Global Cervical Cancer Screening Market Insights, Growth, Share, Size: By Offerings, By Test Type, By Cancer Type, By Age Group, By End Users, By Region & Segmental Forecast, 2023-2031, Comparative Analysis and Trends
- Industry: Healthcare
- Report ID: TNR-110-956
- Number of Pages: 420
- Table/Charts : Yes
- November, 2023
- Base Year : 2024
- No. of Companies : 14+
- No. of Countries : 29
- Views : 10180
- Covid Impact Covered: Yes
- War Impact Covered: Yes
- Formats : PDF, Excel, PPT
Global Cervical Cancer Screening Market was Valued USD 1.4 Bn in 2022, with an Estimated CAGR of 13.7% (2023- 2031)
Cervical cancer screening is a medical process that involves the detection of early signs of cervical cancer or pre-cancerous changes in the cervix (the lower part of the uterus). The primary purpose of cervical cancer screening is to identify abnormalities in cervical cells so that they can be treated before they progress to invasive cervical cancer.
While the rise in cervical cancer screening, it’s essential to maintain and expand these efforts to reach all eligible individuals and reduce the incidence and mortality rates associated with cervical cancer further. Regular screening, combined with vaccination and education, remains a cornerstone of cervical cancer prevention and early detection. Global cervical cancer screening market continues to evolve with advancements in technology, changes in healthcare policies, and increasing awareness of the importance of early detection.
Global Cervical Cancer Screening Market Revenue & Forecast, (US$ Million), 2015 – 2031

Global Cervical Cancer Screening Market Future
The future may see a move towards personalized screening strategies based on an individual’s risk factors and genetics. Tailored screening approaches could help optimize resources and improve early detection rates. Besides continued advancements in screening technologies, such as the development of more sensitive and specific HPV tests and the integration of artificial intelligence (AI) and machine learning in cytology and pathology, may lead to more accurate and efficient screening methods.
There is also a growing interest in home-based cervical cancer screening options, such as self-sampling kits that allow women to collect samples themselves and send them for testing. These options can increase screening participation, especially among underserved populations. Overall, the cervical cancer screening market is expected to evolve with a greater emphasis on precision, accessibility, and patient-centric approaches.
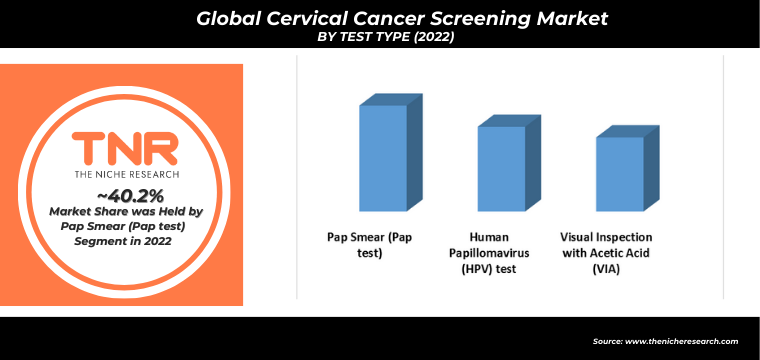
Human Papillomavirus (HPV) test is anticipated to be the fastest growing segment in the cervical cancer screening market. In recent years, human papillomavirus (HPV) testing has gained prominence in cervical cancer screening programs. HPV testing is often used in conjunction with pap smears (co-testing) or as a primary screening method, especially for women aged 30 and older. High-risk HPV strains are strongly associated with cervical cancer.

North America dominated the cervical cancer screening market in 2022. The steepest annual increase in new cases of advanced womb (cervical) cancer in the United States is among white women, who are significantly less likely to receive the preventive HPV (human papillomavirus) vaccine or be screened for the disease, according to new research published International Journal of Gynaecological Cancer.
There has been a significant increase in awareness regarding cervical cancer screening in recent years. With greater usage of the pap test, the mortality rate from cervical cancer decreased dramatically. To prevent HPV infections, North America has well-established HPV vaccination programmes, and screening guidelines are issued by healthcare organisations such as the American Cancer Society (ACS) and the American College of Obstetricians and Gynaecologists (ACOG).
Competitive Landscape
The global cervical cancer screening market is robust with ongoing research and development efforts aimed at improving the accuracy and accessibility of cervical cancer screening. The report offers an in-depth analysis of the competitive landscape which is further influenced by emerging trends, evolving customer demands, strategic partnerships, and acquisitions, making it a dynamic and evolving market.
Leading players operating in the global cervical cancer screening market are:
- Abbott
- BD
- Bio-Rad Laboratories, Inc.
- Cepheid
- Everlywell, Inc.
- F. Hoffmann-La Roche
- FUJIREBIO
- Hologic, Inc.
- LifeCell International
- Promega Corporation
- QIAGEN
- Quest Diagnostics
- Sansure Biotech Inc.
- Sysmex Inostics Inc.
- Thermo Fisher Scientific Inc.
- Other market participants
Global Cervical Cancer Screening Market Report Coverage
| Report Specifications | Details |
| Market Revenue in 2022 | US$ 1.4 Billion |
| Market Size Forecast by 2031 | US$ 4.8 Billion |
| Growth Rate (CAGR) | 13.7% |
| Historic Data | 2015 – 2021 |
| Base Year for Estimation | 2022 |
| Forecast Period | 2023 – 2031 |
| Report Inclusions | Market Size & Estimates, Market Dynamics, Competitive Scenario, Trends, Growth Factors, Market Determinants, Key Investment Segmentation, Product/Service/Solutions Benchmarking |
| Segments Covered | By Offerings, By Test Type, By Cancer Type, By Age Group, By End Users |
| Regions Covered | North America, Europe, Asia Pacific, Middle East & Africa, Latin America |
| Countries Covered | U.S., Canada, Mexico, Rest of North America, France, The UK, Spain, Germany, Italy, Nordic Countries (Denmark, Finland, Iceland, Sweden, Norway), Benelux Union (Belgium, The Netherlands, Luxembourg), Rest of Europe, China, Japan, India, New Zealand, Australia, South Korea, Southeast Asia (Indonesia, Thailand, Malaysia, Singapore, Rest of Southeast Asia), Rest of Asia Pacific, Saudi Arabia, UAE, Egypt, Kuwait, South Africa, Rest of Middle East & Africa, Brazil, Argentina, Rest of Latin America |
| Key Players | Abbott, BD, Bio-Rad Laboratories, Inc., Cepheid, Everlywell, Inc., F. Hoffmann-La Roche, FUJIREBIO, Hologic, Inc. ,LifeCell International, Promega Corporation, QIAGEN, Quest Diagnostics, Sansure Biotech Inc., Sysmex Inostics Inc., Thermo Fisher Scientific Inc., Other Industry Participants |
| Customization Scope | Customization allows for the inclusion/modification of content pertaining to geographical regions, countries, and specific market segments. |
| Pricing & Procurement Options | Explore purchase options tailored to your specific research requirements |
| Contact Details | Consult With Our Expert
Japan (Toll-Free): – +81 663-386-8111 South Korea (Toll-Free): – +82-808- 703-126 Saudi Arabia (Toll-Free): – +966 800 850 1643 United States: +1 302-232-5106 United Kingdom: +447537105080 E-mail: askanexpert@thenicheresearch.com
|
Global Cervical Cancer Screening Market
By Offerings
- Test Kits
- Consumables
By Test Type
- Pap Smear (Pap test)
- Human Papillomavirus (HPV) test
- Visual Inspection with Acetic Acid (VIA)
By Cancer Type
- Squamous cell carcinoma
- Adenocarcinoma
By Age Group
- 21-29 years
- 30-65 years
- Above 65 years
By End User
- Hospitals and clinics
- Cancer research institutes
- Diagnostic Laboratories
- At- Home testing
- Others
By Region
- North America (U.S., Canada, Mexico, Rest of North America)
- Europe (France, The UK, Spain, Germany, Italy, Nordic Countries (Denmark, Finland, Iceland, Sweden, Norway), Benelux Union (Belgium, The Netherlands, Luxembourg), Rest of Europe)
- Asia Pacific (China, Japan, India, New Zealand, Australia, South Korea, Southeast Asia (Indonesia, Thailand, Malaysia, Singapore, Rest of Southeast Asia), Rest of Asia Pacific)
- Middle East & Africa (Saudi Arabia, UAE, Egypt, Kuwait, South Africa, Rest of Middle East & Africa)
- Latin America (Brazil, Argentina, Rest of Latin America)
Report Coverage and Deliverables:
Table of Contents
Note: This ToC is tentative and can be changed according to the research study conducted during the course of report completion.
**Exclusive for Multi-User and Enterprise User.
Global Cervical Cancer Screening Market
By Offerings
By Test Type
By Cancer Type
By Age Group
By End User
By Region
**Note: The report covers cross-segmentation analysis by region further into countries
The Niche Research approach encompasses both primary and secondary research methods to provide comprehensive insights. While primary research is the cornerstone of our studies, we also incorporate secondary research sources such as company annual reports, premium industry databases, press releases, industry journals, and white papers.
Within our primary research, we actively engage with various industry stakeholders, conducting paid interviews and surveys. Our meticulous analysis extends to every market participant in major countries, allowing us to thoroughly examine their portfolios, calculate market shares, and segment revenues.
Our data collection primarily focuses on individual countries within our research scope, enabling us to estimate regional market sizes. Typically, we employ a bottom-up approach, meticulously tracking trends in different countries. We analyze growth drivers, constraints, technological innovations, and opportunities for each country, ultimately arriving at regional figures.Our process begins by examining the growth prospects of each country. Building upon these insights, we project growth and trends for the entire region. Finally, we utilize our proprietary model to refine estimations and forecasts.
Our data validation standards are integral to ensuring the reliability and accuracy of our research findings. Here’s a breakdown of our data validation processes and the stakeholders we engage with during our primary research:
- Supply Side Analysis: We initiate a supply side analysis by directly contacting market participants, through telephonic interviews and questionnaires containing both open-ended and close-ended questions. We gather information on their portfolios, segment revenues, developments, and growth strategies.
- Demand Side Analysis: To gain insights into adoption trends and consumer preferences, we reach out to target customers and users (non-vendors). This information forms a vital part of the qualitative analysis section of our reports, covering market dynamics, adoption trends, consumer behavior, spending patterns, and other related aspects.
- Consultant Insights: We tap into the expertise of our partner consultants from around the world to obtain their unique viewpoints and perspectives. Their insights contribute to a well-rounded understanding of the markets under investigation.
- In-House Validation: To ensure data accuracy and reliability, we conduct cross-validation of data points and information through our in-house team of consultants and utilize advanced data modeling tools for thorough verification.
The forecasts we provide are based on a comprehensive assessment of various factors, including:
- Market Trends and Past Performance (Last Five Years): We accurately analyze market trends and performance data from preceding five years to identify historical patterns and understand the market’s evolution.
- Historical Performance and Growth of Market Participants: We assess the historical performance and growth trajectories of key market participants. This analysis provides insights into the competitive landscape and individual company strategies.
- Market Determinants Impact Analysis (Next Eight Years): We conduct a rigorous analysis of the factors that are projected to influence the market over the next eight years. This includes assessing both internal and external determinants that can shape market dynamics.
- Drivers and Challenges for the Forecast Period:Identify the factors expected to drive market growth during the forecast period, as well as the challenges that the industry may face. This analysis aids in deriving an accurate growth rate projection.
- New Acquisitions, Collaborations, or Partnerships: We keep a close watch on any new acquisitions, collaborations, or partnerships within the industry. These developments can have a significant impact on market dynamics and competitiveness.
- Macro and Micro Factors Analysis:A thorough examination of both macro-level factors (e.g., economic trends, regulatory changes) and micro-level factors (e.g., technological advancements, consumer preferences) that may influence the market during the forecast period.
- End-User Sentiment Analysis: To understand the market from the end-user perspective, we conduct sentiment analysis. This involves assessing the sentiment, preferences, and feedback of the end-users, which can provide valuable insights into market trends.
- Perspective of Primary Participants: Insights gathered directly from primary research participants play a crucial role in shaping our forecasts. Their perspectives and experiences provide valuable qualitative data.
- Year-on-Year Growth Trend: We utilize a year-on-year growth trend based on historical market growth and expected future trends. This helps in formulating our growth projections, aligning them with the market’s historical performance.
Research process adopted by TNR involves multiple stages, including data collection, validation, quality checks, and presentation. It’s crucial that the data and information we provide add value to your existing market understanding and expertise. We have also established partnerships with business consulting, research, and survey organizations across regions and globally to collaborate on regional analysis and data validation, ensuring the highest level of accuracy and reliability in our reports.









