Global CMO/CDMO Market By Product Type, By Therapeutic Area, By End Use Industry, By Region & Segmental Insights Trends and Forecast, 2024 – 2034
- Industry: Healthcare
- Report ID: TNR-110-1059
- Number of Pages: 420
- Table/Charts : Yes
- March, 2024
- Base Year : 2024
- No. of Companies : 10+
- No. of Countries : 29
- Views : 10195
- Covid Impact Covered: Yes
- War Impact Covered: Yes
- Formats : PDF, Excel, PPT
Contract Manufacturing Organization (CMO) and Contract Development and Manufacturing Organization (CDMO) market play pivotal roles in the pharmaceutical, biotechnology, and healthcare industries. These organizations offer a range of services, from drug development and formulation to manufacturing and packaging. The market has witnessed significant growth due to several factors, including increasing outsourcing trends by pharmaceutical companies, cost-saving initiatives, and the complexity of drug development processes.
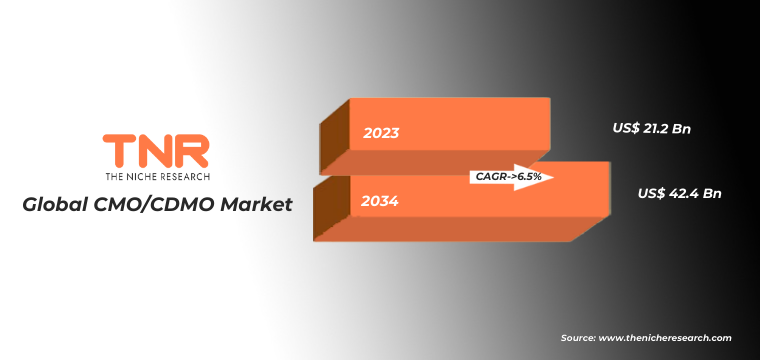
CMOs-CDMOs provide flexibility, expertise, and specialized infrastructure, allowing pharmaceutical companies to focus on core competencies such as research and marketing. Moreover, the expanding biopharmaceutical sector, coupled with the demand for personalized medicines, has further fuelled the market’s growth. In terms of revenue, the global CMO/CDMO market was worth US$ 21.2 Bn in 2023 and is anticipated to witness a CAGR of 6.5% during 2024 – 2034.
Global CMO/CDMO Market Revenue & Forecast, (US$ Million), 2016 – 2034
Trends in the Global CMO-CDMO Market
- A notable trend in the global CMO/CDMO market is the increasing focus on personalized medicine. With advancements in genomics and targeted therapies, pharmaceutical companies are seeking CMOs/CDMOs capable of delivering tailored drug development and manufacturing solutions. This trend reflects the shift towards individualized treatments, driving demand for flexible manufacturing processes and technologies that can accommodate small batch sizes and rapid scale-up capabilities.
- Pharmaceutical companies are outsourcing tasks such as nanomedicines, lipid-based formulations, and controlled-release formulations to leverage the specialized expertise and infrastructure offered by CMOs/CDMOs. This trend allows pharmaceutical companies to access advanced technologies and reduce time-to-market for innovative drug products while mitigating the risks associated with in-house development of complex formulations.
Manufacturing services type dominated the global market during the forecast period. Pharmaceutical companies increasingly outsource manufacturing activities to CMOs/CDMOs to capitalize on their specialized expertise and infrastructure, allowing them to focus on core competencies like research and development.
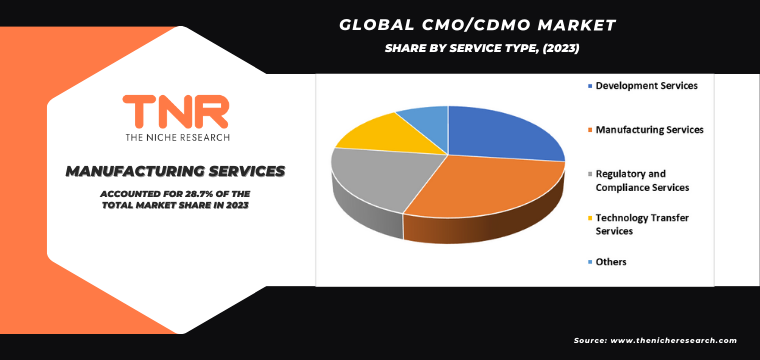
Additionally, the growing complexity of drug manufacturing processes, particularly for biologics and advanced therapies, necessitates the involvement of CMOs/CDMOs with advanced technologies and regulatory compliance. Also, the cost-effectiveness and scalability offered by outsourcing manufacturing services contribute to the preference for CMO/CDMO partnerships. As a result, drug substance manufacturing is expected to remain the dominant segment in the CMO/CDMO market, driving its growth during the forecast period.
By therapeutic area, the infectious diseases segment gained popularity in recent years and is anticipated to grow the fastest over the forecast timeline. The emergence of new infectious pathogens and the re-emergence of previously controlled diseases have heightened global awareness and investment in combating infectious diseases. Events such as the COVID-19 pandemic have underscored the critical need for rapid response capabilities, leading to increased demand for contract manufacturing organizations (CMOs) and contract development and manufacturing organizations (CDMOs) specializing in infectious disease therapeutics.

Moreover, advancements in biotechnology and molecular biology have facilitated the development of novel therapeutics and vaccines targeting infectious agents. This has spurred pharmaceutical companies and biotech’s to outsource manufacturing processes to specialized CMOs/CDMOs with the requisite expertise and infrastructure.

The pharmaceuticals segment by end use industry accounted for the largest revenue share in 2023 in the global market. The increasing complexity of drug development processes, coupled with stringent regulatory requirements, has led pharmaceutical companies to seek specialized expertise and infrastructure offered by CMOs/CDMOs. Additionally, the rising demand for personalized medicine and biologics has fuelled the need for flexible manufacturing capacities provided by outsourcing partners.
Furthermore, cost-saving strategies, operational efficiencies, and focus on core competencies have incentivized pharmaceutical companies to collaborate with CMOs/CDMOs for various stages of drug development, from formulation to commercial production. These factors collectively contribute to the prominence of the pharmaceuticals segment in the global CMO/CDMO market.
Asia Pacific is expected to emerge as the second-leading region in the CMO/CDMO Market, following North America. The region boasts a rapidly expanding pharmaceutical and biotechnology industry, driven by increasing healthcare expenditure, a growing population, and rising demand for innovative therapies. Secondly, Asia Pacific offers a competitive advantage in terms of cost-effective manufacturing capabilities, skilled workforce, and robust regulatory frameworks conducive to outsourcing partnerships. Additionally, strategic investments in infrastructure and technology advancements have further strengthened the region’s position as a preferred destination for CMO/CDMO services.
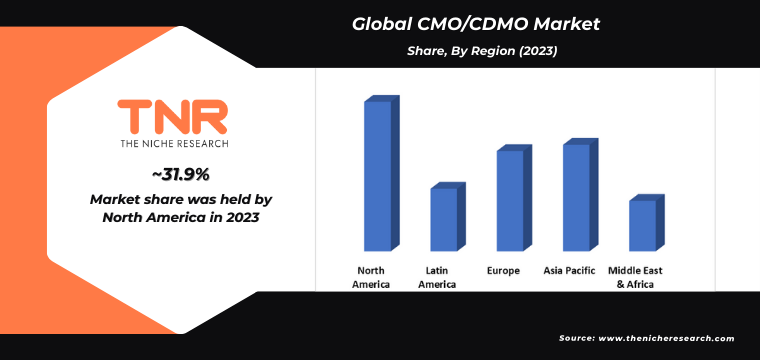
Moreover, favourable government policies, such as tax incentives and streamlined approval processes, attract multinational pharmaceutical companies to establish partnerships and manufacturing facilities in the region, contributing to its emergence as a key player in the global CMO/CDMO market.
Competitive Landscape
Major players such as Lonza Group, Thermo Fisher Scientific, and Catalent, Inc. dominate the market with extensive service offerings and global presence. Additionally, there is a growing presence of regional players and niche service providers catering to specific therapeutic areas or manufacturing technologies, fostering competition and innovation within the industry.
Some of the players operating in the CMO/CDMO Market are
- AXXELENT
- BDR Pharmaceuticals Internationals Pvt. Ltd.
- Catalent
- CoreRx
- Eurofins Scientific
- HIKAL Ltd.
- Idifarma
- KBI Biopharma.
- Laurus Synthesis
- Lonza
- mAbxience
- Piramal Pharma Solutions
- QuayPharma
- Sai Life Sciences
- Thermo Fisher Scientific
- Other Industry Participants
Global CMO/CDMO Market: Key Insights
| Report Specifications | Details |
| Market Revenue in 2023 | US$ 21.2 Bn |
| Market Size Forecast by 2034 | US$ 42.4 Bn |
| Growth Rate (CAGR) | 6.5% |
| Historic Data | 2016 – 2022 |
| Base Year for Estimation | 2023 |
| Forecast Period | 2024 – 2034 |
| Report Inclusions | Market Size & Estimates, Market Dynamics, Competitive Scenario, Trends, Growth Factors, Market Determinants, Key Investment Segmentation, Product/Service/Solutions Benchmarking |
| Segments Covered | By Product Type, By Therapeutic Area, By End Use Industry |
| Regions Covered | North America, Europe, Asia Pacific, Middle East & Africa, Latin America |
| Countries Covered | U.S., Canada, Mexico, Rest of North America, France, The UK, Spain, Germany, Italy, Nordic Countries (Denmark, Finland, Iceland, Sweden, Norway), Benelux Union (Belgium, The Netherlands, Luxembourg), Rest of Europe, China, Japan, India, New Zealand, Australia, South Korea, Southeast Asia (Indonesia, Thailand, Malaysia, Singapore, Rest of Southeast Asia), Rest of Asia Pacific, Saudi Arabia, UAE, Egypt, Kuwait, South Africa, Rest of Middle East & Africa, Brazil, Argentina, Rest of Latin America |
| Key Players | AXXELENT, BDR Pharmaceuticals Internationals Pvt. Ltd., Catalent, CoreRx, Eurofins Scientific, HIKAL Ltd., Idifarma, KBI Biopharma., Laurus Synthesis, Lonza, mAbxience, Piramal Pharma Solutions, QuayPharma, Sai Life Sciences, Thermo Fisher Scientific, Other Market Participants |
| Customization Scope | Customization allows for the inclusion/modification of content pertaining to geographical regions, countries, and specific market segments. |
| Pricing & Procurement Options | Explore purchase options tailored to your specific research requirements |
| Contact Details | Consult With Our Expert
Japan (Toll-Free): +81 663-386-8111 South Korea (Toll-Free): +82-808- 703-126 Saudi Arabia (Toll-Free): +966 800-850-1643 United Kingdom: +44 753-710-5080 United States: +1 302-232-5106 E-mail: askanexpert@thenicheresearch.com
|
Global CMO/CDMO Market
By Service Type
- Development Services
- Formulation Development
- Process Development
- Analytical Development
- Preclinical and Clinical Manufacturing
- Manufacturing Services
- API Manufacturing
- Drug Product Manufacturing
- Biologics Manufacturing
- Medical Device Manufacturing
- Packaging and Labelling
- Supply Chain Management
- Regulatory and Compliance Services
- Regulatory Consulting
- Quality Assurance and Control
- Validation Services
- Technology Transfer Services
- Others
By Therapeutic Area
- Oncology
- Neurology
- Cardiovascular
- Infectious Diseases
- Autoimmune Disorders
- Others
By End Use Industry
- Pharmaceuticals
- Biotechnology
- Medical Devices
- Others
By Region
- North America (U.S., Canada, Mexico, Rest of North America)
- Europe (France, The UK, Spain, Germany, Italy, Nordic Countries (Denmark, Finland, Iceland, Sweden, Norway), Benelux Union (Belgium, The Netherlands, Luxembourg), Rest of Europe)
- Asia Pacific (China, Japan, India, New Zealand, Australia, South Korea, Southeast Asia (Indonesia, Thailand, Malaysia, Singapore, Rest of Southeast Asia), Rest of Asia Pacific)
- Middle East & Africa (Saudi Arabia, UAE, Egypt, Kuwait, South Africa, Rest of Middle East & Africa)
- Latin America (Brazil, Argentina, Rest of Latin America)
Report Coverage

Table of Contents
Note: This ToC is tentative and can be changed according to the research study conducted during the course of report completion.
**Exclusive for Multi-User and Enterprise User.
Global CMO/CDMO Market
By Service Type
- Development Services
- Formulation Development
- Process Development
- Analytical Development
- Preclinical and Clinical Manufacturing
- Manufacturing Services
- API Manufacturing
- Drug Product Manufacturing
- Biologics Manufacturing
- Medical Device Manufacturing
- Packaging and Labelling
- Supply Chain Management
- Regulatory and Compliance Services
- Regulatory Consulting
- Quality Assurance and Control
- Validation Services
- Technology Transfer Services
- Others
By Therapeutic Area
- Oncology
- Neurology
- Cardiovascular
- Infectious Diseases
- Autoimmune Disorders
- Others
By End Use Industry
- Pharmaceuticals
- Biotechnology
- Medical Devices
- Others
By Region
- North America (U.S., Canada, Mexico, Rest of North America)
- Europe (France, The UK, Spain, Germany, Italy, Nordic Countries (Denmark, Finland, Iceland, Sweden, Norway), Benelux Union (Belgium, The Netherlands, Luxembourg), Rest of Europe)
- Asia Pacific (China, Japan, India, New Zealand, Australia, South Korea, Southeast Asia (Indonesia, Thailand, Malaysia, Singapore, Rest of Southeast Asia), Rest of Asia Pacific)
- Middle East & Africa (Saudi Arabia, UAE, Egypt, Kuwait, South Africa, Rest of Middle East & Africa)
- Latin America (Brazil, Argentina, Rest of Latin America)
The Niche Research approach encompasses both primary and secondary research methods to provide comprehensive insights. While primary research is the cornerstone of our studies, we also incorporate secondary research sources such as company annual reports, premium industry databases, press releases, industry journals, and white papers.
Within our primary research, we actively engage with various industry stakeholders, conducting paid interviews and surveys. Our meticulous analysis extends to every market participant in major countries, allowing us to thoroughly examine their portfolios, calculate market shares, and segment revenues.
Our data collection primarily focuses on individual countries within our research scope, enabling us to estimate regional market sizes. Typically, we employ a bottom-up approach, meticulously tracking trends in different countries. We analyze growth drivers, constraints, technological innovations, and opportunities for each country, ultimately arriving at regional figures.Our process begins by examining the growth prospects of each country. Building upon these insights, we project growth and trends for the entire region. Finally, we utilize our proprietary model to refine estimations and forecasts.
Our data validation standards are integral to ensuring the reliability and accuracy of our research findings. Here’s a breakdown of our data validation processes and the stakeholders we engage with during our primary research:
- Supply Side Analysis: We initiate a supply side analysis by directly contacting market participants, through telephonic interviews and questionnaires containing both open-ended and close-ended questions. We gather information on their portfolios, segment revenues, developments, and growth strategies.
- Demand Side Analysis: To gain insights into adoption trends and consumer preferences, we reach out to target customers and users (non-vendors). This information forms a vital part of the qualitative analysis section of our reports, covering market dynamics, adoption trends, consumer behavior, spending patterns, and other related aspects.
- Consultant Insights: We tap into the expertise of our partner consultants from around the world to obtain their unique viewpoints and perspectives. Their insights contribute to a well-rounded understanding of the markets under investigation.
- In-House Validation: To ensure data accuracy and reliability, we conduct cross-validation of data points and information through our in-house team of consultants and utilize advanced data modeling tools for thorough verification.
The forecasts we provide are based on a comprehensive assessment of various factors, including:
- Market Trends and Past Performance (Last Five Years): We accurately analyze market trends and performance data from preceding five years to identify historical patterns and understand the market’s evolution.
- Historical Performance and Growth of Market Participants: We assess the historical performance and growth trajectories of key market participants. This analysis provides insights into the competitive landscape and individual company strategies.
- Market Determinants Impact Analysis (Next Eight Years): We conduct a rigorous analysis of the factors that are projected to influence the market over the next eight years. This includes assessing both internal and external determinants that can shape market dynamics.
- Drivers and Challenges for the Forecast Period:Identify the factors expected to drive market growth during the forecast period, as well as the challenges that the industry may face. This analysis aids in deriving an accurate growth rate projection.
- New Acquisitions, Collaborations, or Partnerships: We keep a close watch on any new acquisitions, collaborations, or partnerships within the industry. These developments can have a significant impact on market dynamics and competitiveness.
- Macro and Micro Factors Analysis:A thorough examination of both macro-level factors (e.g., economic trends, regulatory changes) and micro-level factors (e.g., technological advancements, consumer preferences) that may influence the market during the forecast period.
- End-User Sentiment Analysis: To understand the market from the end-user perspective, we conduct sentiment analysis. This involves assessing the sentiment, preferences, and feedback of the end-users, which can provide valuable insights into market trends.
- Perspective of Primary Participants: Insights gathered directly from primary research participants play a crucial role in shaping our forecasts. Their perspectives and experiences provide valuable qualitative data.
- Year-on-Year Growth Trend: We utilize a year-on-year growth trend based on historical market growth and expected future trends. This helps in formulating our growth projections, aligning them with the market’s historical performance.
Research process adopted by TNR involves multiple stages, including data collection, validation, quality checks, and presentation. It’s crucial that the data and information we provide add value to your existing market understanding and expertise. We have also established partnerships with business consulting, research, and survey organizations across regions and globally to collaborate on regional analysis and data validation, ensuring the highest level of accuracy and reliability in our reports.









