Global Active Pharmaceutical Ingredient Market: By Type, By Synthesis, By Type of Drug, By Potency, By Therapeutic Applications, By End User, By Region & Segmental Insights Trends and Forecast, 2024 – 2034
- Industry: Healthcare
- Report ID: TNR-110-1146
- Number of Pages: 420
- Table/Charts : Yes
- June, 2024
- Base Year : 2024
- No. of Companies : 10+
- No. of Countries : 29
- Views : 10141
- Covid Impact Covered: Yes
- War Impact Covered: Yes
- Formats : PDF, Excel, PPT
An active pharmaceutical ingredient (API) is a crucial component in the formulation of medications, responsible for the therapeutic effects and efficacy of the drug. APIs are the biologically active elements within a pharmaceutical product that interact with the body to diagnose, treat, cure, or prevent disease. The quality and potency of an API directly influence the effectiveness and safety of the medication. APIs can be derived from various sources, including chemical synthesis, biotechnology, and natural extraction, depending on the nature and complexity of the drug.
The production of APIs involves stringent regulatory standards and quality control measures to ensure purity and consistency. Advances in pharmaceutical technology and biotechnology have enabled the development of highly specialized APIs, such as those used in targeted therapies and biologics, enhancing the capability to treat complex and chronic conditions. The API industry is a foundational element of the pharmaceutical supply chain, critical to the development of effective medical treatments.
The demand for active pharmaceutical ingredients (APIs) is driven by several critical factors shaping the pharmaceutical landscape. One primary driver is the increasing prevalence of chronic diseases such as diabetes, cancer, and cardiovascular conditions, necessitating a continuous supply of effective medications. Additionally, the aging global population requires more pharmaceutical interventions, further boosting API demand.
The rise in biopharmaceutical innovations, including biologics and personalized medicine, relies heavily on advanced APIs tailored to specific therapeutic needs. Regulatory incentives for the development of innovative drugs and the expansion of generic medications due to patent expirations also contribute to the growing API market. Moreover, the trend toward outsourcing API production to cost-effective regions enhances global supply chain efficiency, meeting the rising healthcare needs. These dynamics collectively propel the demand for high-quality APIs, underscoring their essential role in modern healthcare and pharmaceutical advancements.
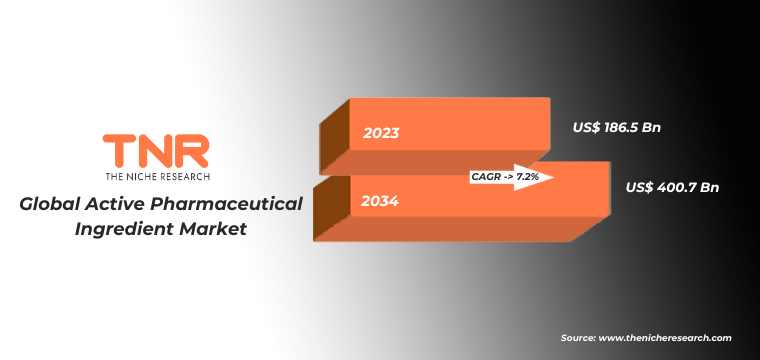
In terms of revenue, the global active pharmaceutical ingredient market was worth US$ 186.5 Bn in 2023, anticipated to witness CAGR of 7.2% during 2024 – 2034.
Global Active Pharmaceutical Ingredient Market Dynamics
Rising Prevalence of Chronic Diseases: The increasing incidence of chronic diseases such as cancer, diabetes, and cardiovascular disorders fuels the demand for APIs, as these conditions require ongoing medication management. A growing elderly population worldwide leads to a higher demand for medications, driving the need for diverse and effective APIs.
Advancements in Biotechnology: Innovations in biotechnology, such as the development of biologics and biosimilars, significantly boost the demand for complex and specialized APIs. Favorable government policies and regulatory frameworks that encourage pharmaceutical innovation and expedite drug approval processes contribute to market growth.
Generic Drug Market Expansion: The expiration of patents for blockbuster drugs opens up opportunities for generic API manufacturers, increasing the availability and affordability of medications. Pharmaceutical companies increasingly outsource API production to specialized manufacturers, often in regions with lower production costs, enhancing global supply chain efficiency.
Technological Advancements: Improvements in API manufacturing processes, such as green chemistry and continuous manufacturing, enhance production efficiency, reduce costs, and ensure higher quality standards. Economic growth in emerging markets increases healthcare spending and access to medications, further expanding the API market.
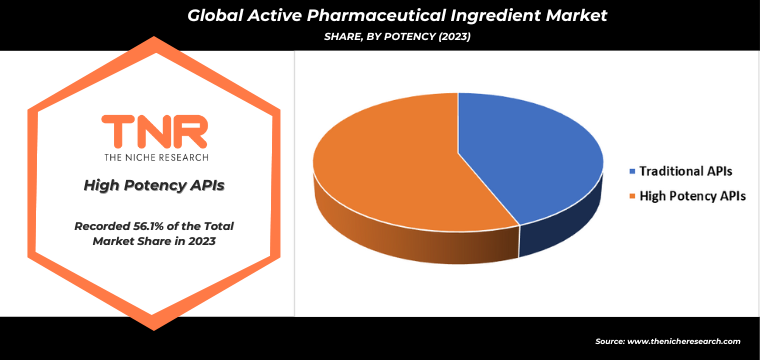
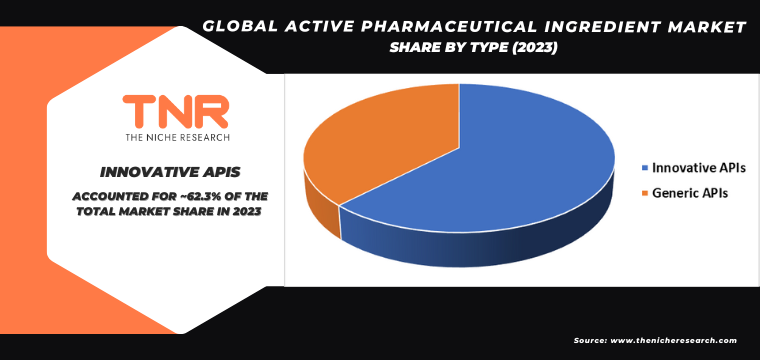
Innovative API Segment Has Garnered Major Market Share in the Global Active Pharmaceutical Ingredient Market During the Forecast Period (2024 – 2034).
The demand for innovative active pharmaceutical ingredients (APIs) is driven by the constant evolution and advancement in the pharmaceutical industry. Innovative APIs, often resulting from cutting-edge research and development, offer enhanced efficacy, reduced side effects, and novel mechanisms of action, addressing unmet medical needs and improving patient outcomes. The rise of personalized medicine, which tailors treatments based on individual genetic profiles, necessitates the development of new APIs that can specifically target unique biomarkers and pathways.
Additionally, the growing prevalence of chronic diseases and the continuous emergence of new infectious diseases push for the creation of more effective and advanced therapeutic options. Biopharmaceutical advancements, including gene therapy and monoclonal antibodies, also rely heavily on innovative APIs. Regulatory incentives and fast-track approvals for breakthrough therapies further stimulate the development and commercialization of these APIs, ensuring that the pharmaceutical market continues to innovate and expand, meeting the increasing global demand for sophisticated medical treatments.
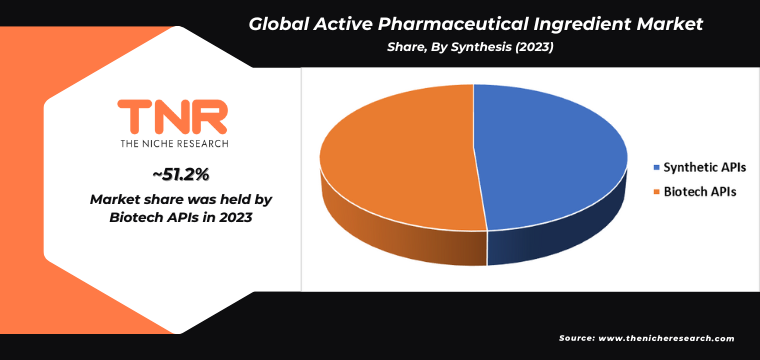
Mammalian Expression Systems Segment had the Highest Share in the Global Active Pharmaceutical Ingredient Market in 2023.
The increasing demand for active pharmaceutical ingredients (APIs) is significantly propelled by the advancements and utilization of mammalian expression systems in biopharmaceutical production. These systems are essential for producing complex, high-quality proteins and monoclonal antibodies that are critical for treating a wide range of diseases, including cancer, autoimmune disorders, and infectious diseases. Mammalian cells, such as CHO (Chinese Hamster Ovary) cells, are preferred due to their ability to perform intricate post-translational modifications, ensuring the biological activity and efficacy of therapeutic proteins.
The surge in biologics and personalized medicine further amplifies the need for these sophisticated expression systems, as they can be tailored to produce specific human proteins with high fidelity. Additionally, regulatory agencies increasingly recognize and approve biologics derived from mammalian systems, reinforcing their role in modern pharmaceutical manufacturing. Consequently, the reliance on mammalian expression systems is a pivotal driver in the growing demand for APIs.
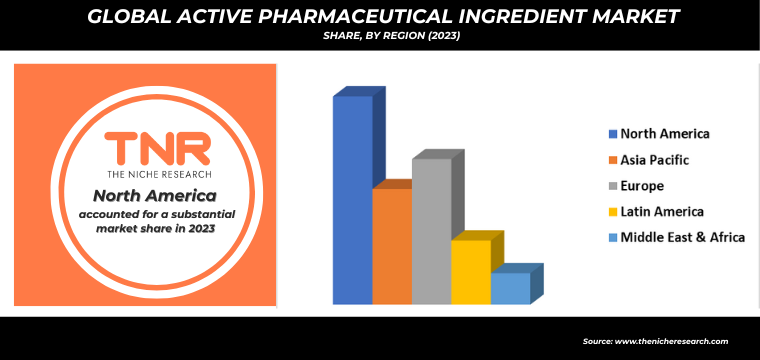
By Region, North America Dominated the Global Active Pharmaceutical Ingredient Market in 2023.
The demand for active pharmaceutical ingredients (APIs) in North America is driven by several key factors, reflecting the region’s robust healthcare infrastructure and innovation capabilities. Foremost among these drivers is the aging population, which necessitates an increased production of medications for chronic and age-related conditions. Additionally, the rise in prevalence of lifestyle diseases such as diabetes, cardiovascular disorders, and obesity requires a steady supply of APIs for a range of treatments.
The strong emphasis on research and development within the pharmaceutical industry also fuels demand, as new drug formulations and biopharmaceuticals emerge. Furthermore, the COVID-19 pandemic has highlighted the need for robust supply chains and domestic manufacturing of critical drug components, prompting investments in API production. Regulatory support and initiatives to reduce dependency on imports, alongside advancements in biotechnology, are also crucial in sustaining the growing demand for APIs in North America.
Competitive Landscape: Global Active Pharmaceutical Ingredient Market:
- Abbott
- AbbVie Inc.
- Amgen Inc.
- Aurobindo Pharma
- Biocon
- Boehringer Ingelheim International GmbH
- Cipla Inc.
- Reddy’s Laboratories Ltd
- Mylan N.V.
- Sun Pharmaceutical Industries Ltd
- Teva Pharmaceutical Industries Ltd
- Other Industry Participants
Global Active Pharmaceutical Ingredient Market Scope
| Report Specifications | Details |
| Market Revenue in 2023 | US$ 186.5 Bn |
| Market Size Forecast by 2034 | US$ 400.7 Bn |
| Growth Rate (CAGR) | 7.2% |
| Historic Data | 2016 – 2022 |
| Base Year for Estimation | 2023 |
| Forecast Period | 2024 – 2034 |
| Report Inclusions | Market Size & Estimates, Market Dynamics, Competitive Scenario, Trends, Growth Factors, Market Determinants, Key Investment Segmentation, Product/Service/Solutions Benchmarking |
| Segments Covered | By Type, By Synthesis, By Type of Drug, By Potency, By Therapeutic Applications, By End User, By Region |
| Regions Covered | North America, Europe, Asia Pacific, Middle East & Africa, Latin America |
| Countries Covered | U.S., Canada, Mexico, Rest of North America, France, The UK, Spain, Germany, Italy, Nordic Countries (Denmark, Finland, Iceland, Sweden, Norway), Benelux Union (Belgium, The Netherlands, Luxembourg), Rest of Europe, China, Japan, India, New Zealand, Australia, South Korea, Southeast Asia (Indonesia, Thailand, Malaysia, Singapore, Rest of Southeast Asia), Rest of Asia Pacific, Saudi Arabia, UAE, Egypt, Kuwait, South Africa, Rest of Middle East & Africa, Brazil, Argentina, Rest of Latin America |
| Key Players | Abbott, AbbVie Inc., Amgen Inc., Aurobindo Pharma, Biocon, Boehringer Ingelheim International GmbH, Cipla Inc., Dr. Reddy’s Laboratories Ltd, Mylan N.V., Sun Pharmaceutical Industries Ltd, Teva Pharmaceutical Industries Ltd |
| Customization Scope | Customization allows for the inclusion/modification of content pertaining to geographical regions, countries, and specific market segments. |
| Pricing & Procurement Options | Explore purchase options tailored to your specific research requirements |
| Contact Details | Consult With Our Expert
Japan (Toll-Free): +81 663-386-8111 South Korea (Toll-Free): +82-808- 703-126 Saudi Arabia (Toll-Free): +966 800-850-1643 United Kingdom: +44 753-710-5080 United States: +1 302-232-5106 E-mail: askanexpert@thenicheresearch.com
|
Global Active Pharmaceutical Ingredient Market
By Type
- Innovative APIs
- Generic APIs
By Synthesis
- Synthetic APIs
- Innovative Synthetic APIs
- Generic Synthetic APIs
- Biotech APIs
- By Type
- Innovative Biotech APIs
- Generic Biotech APIs
- By Product
- Monoclonal Antibodies
- Hormones and Growth Factors
- Fusion proteins
- Cytokines
- Therapeutic enzymes
- Blood factors and Anti-coagulants
- Recombinant vaccines
- By Expression Systems
- Mammalian expression systems
- Microbial expression systems
- Yeast expression systems
- Insect expression system
- Other
- By Type
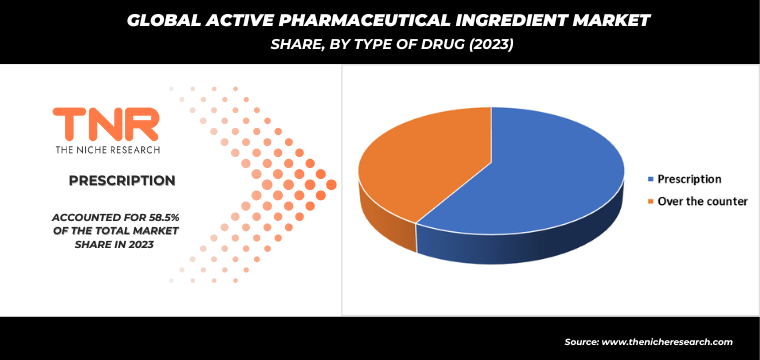
By Type of Drug
- Prescription
- Over the counter
By Potency
- Traditional APIs
- High Potency APIs
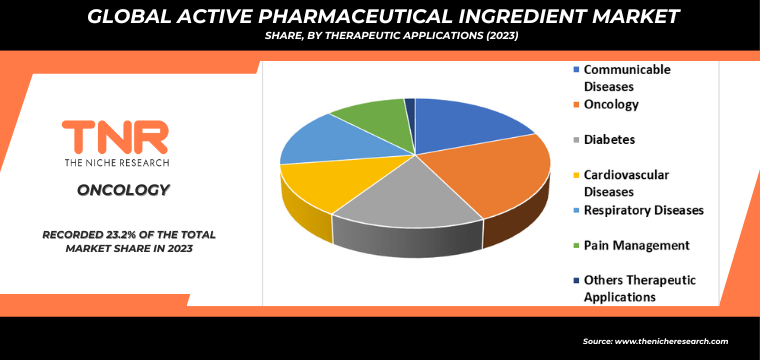
By Therapeutic Applications
- Communicable Diseases
- Oncology
- Diabetes
- Cardiovascular Diseases
- Respiratory Diseases
- Pain Management
- Others Therapeutic Applications
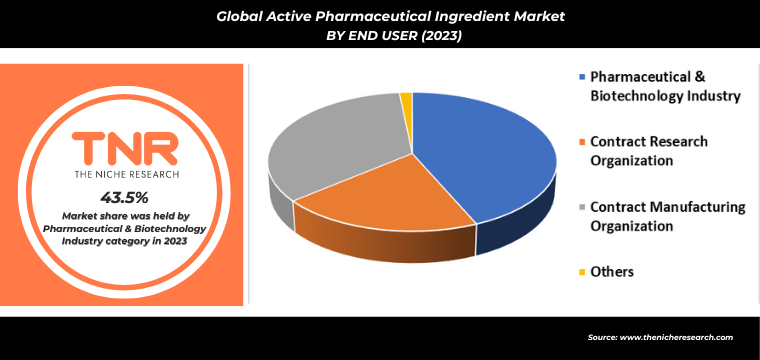
By End User
- Pharmaceutical & Biotechnology Industry
- Contract Research Organization
- Contract manufacturing Organization
- Others
By Region
- North America (U.S., Canada, Mexico, Rest of North America)
- Europe (France, The UK, Spain, Germany, Italy, Nordic Countries (Denmark, Finland, Iceland, Sweden, Norway), Benelux Union (Belgium, The Netherlands, Luxembourg), Rest of Europe)
- Asia Pacific (China, Japan, India, New Zealand, Australia, South Korea, Southeast Asia (Indonesia, Thailand, Malaysia, Singapore, Rest of Southeast Asia), Rest of Asia Pacific)
- Middle East & Africa (Saudi Arabia, UAE, Egypt, Kuwait, South Africa, Rest of Middle East & Africa)
- Latin America (Brazil, Argentina, Rest of Latin America)
Report Layout:
Table of Contents
Note: This ToC is tentative and can be changed according to the research study conducted during the course of report completion.
**Exclusive for Multi-User and Enterprise User.
Global Active Pharmaceutical Ingredient Market
By Type
- Innovative APIs
- Generic APIs
By Synthesis
- Synthetic APIs
- Innovative Synthetic APIs
- Generic Synthetic APIs
- Biotech APIs
- By Type
- Innovative Biotech APIs
- Generic Biotech APIs
- By Product
- Monoclonal Antibodies
- Hormones and Growth Factors
- Fusion proteins
- Cytokines
- Therapeutic enzymes
- Blood factors and Anti-coagulants
- Recombinant vaccines
- By Expression Systems
- Mammalian expression systems
- Microbial expression systems
- Yeast expression systems
- Insect expression system
- Other
- By Type
By Type of Drug
- Prescription
- Over the counter
By Potency
- Traditional APIs
- High Potency APIs
By Therapeutic Applications
- Communicable Diseases
- Oncology
- Diabetes
- Cardiovascular Diseases
- Respiratory Diseases
- Pain Management
- Others Therapeutic Applications
By End User
- Pharmaceutical & Biotechnology Industry
- Contract Research Organization
- Contract manufacturing Organization
- Others
By Region
- North America (U.S., Canada, Mexico, Rest of North America)
- Europe (France, The UK, Spain, Germany, Italy, Nordic Countries (Denmark, Finland, Iceland, Sweden, Norway), Benelux Union (Belgium, The Netherlands, Luxembourg), Rest of Europe)
- Asia Pacific (China, Japan, India, New Zealand, Australia, South Korea, Southeast Asia (Indonesia, Thailand, Malaysia, Singapore, Rest of Southeast Asia), Rest of Asia Pacific)
- Middle East & Africa (Saudi Arabia, UAE, Egypt, Kuwait, South Africa, Rest of Middle East & Africa)
- Latin America (Brazil, Argentina, Rest of Latin America)
The Niche Research approach encompasses both primary and secondary research methods to provide comprehensive insights. While primary research is the cornerstone of our studies, we also incorporate secondary research sources such as company annual reports, premium industry databases, press releases, industry journals, and white papers.
Within our primary research, we actively engage with various industry stakeholders, conducting paid interviews and surveys. Our meticulous analysis extends to every market participant in major countries, allowing us to thoroughly examine their portfolios, calculate market shares, and segment revenues.
Our data collection primarily focuses on individual countries within our research scope, enabling us to estimate regional market sizes. Typically, we employ a bottom-up approach, meticulously tracking trends in different countries. We analyze growth drivers, constraints, technological innovations, and opportunities for each country, ultimately arriving at regional figures.Our process begins by examining the growth prospects of each country. Building upon these insights, we project growth and trends for the entire region. Finally, we utilize our proprietary model to refine estimations and forecasts.
Our data validation standards are integral to ensuring the reliability and accuracy of our research findings. Here’s a breakdown of our data validation processes and the stakeholders we engage with during our primary research:
- Supply Side Analysis: We initiate a supply side analysis by directly contacting market participants, through telephonic interviews and questionnaires containing both open-ended and close-ended questions. We gather information on their portfolios, segment revenues, developments, and growth strategies.
- Demand Side Analysis: To gain insights into adoption trends and consumer preferences, we reach out to target customers and users (non-vendors). This information forms a vital part of the qualitative analysis section of our reports, covering market dynamics, adoption trends, consumer behavior, spending patterns, and other related aspects.
- Consultant Insights: We tap into the expertise of our partner consultants from around the world to obtain their unique viewpoints and perspectives. Their insights contribute to a well-rounded understanding of the markets under investigation.
- In-House Validation: To ensure data accuracy and reliability, we conduct cross-validation of data points and information through our in-house team of consultants and utilize advanced data modeling tools for thorough verification.
The forecasts we provide are based on a comprehensive assessment of various factors, including:
- Market Trends and Past Performance (Last Five Years): We accurately analyze market trends and performance data from preceding five years to identify historical patterns and understand the market’s evolution.
- Historical Performance and Growth of Market Participants: We assess the historical performance and growth trajectories of key market participants. This analysis provides insights into the competitive landscape and individual company strategies.
- Market Determinants Impact Analysis (Next Eight Years): We conduct a rigorous analysis of the factors that are projected to influence the market over the next eight years. This includes assessing both internal and external determinants that can shape market dynamics.
- Drivers and Challenges for the Forecast Period:Identify the factors expected to drive market growth during the forecast period, as well as the challenges that the industry may face. This analysis aids in deriving an accurate growth rate projection.
- New Acquisitions, Collaborations, or Partnerships: We keep a close watch on any new acquisitions, collaborations, or partnerships within the industry. These developments can have a significant impact on market dynamics and competitiveness.
- Macro and Micro Factors Analysis:A thorough examination of both macro-level factors (e.g., economic trends, regulatory changes) and micro-level factors (e.g., technological advancements, consumer preferences) that may influence the market during the forecast period.
- End-User Sentiment Analysis: To understand the market from the end-user perspective, we conduct sentiment analysis. This involves assessing the sentiment, preferences, and feedback of the end-users, which can provide valuable insights into market trends.
- Perspective of Primary Participants: Insights gathered directly from primary research participants play a crucial role in shaping our forecasts. Their perspectives and experiences provide valuable qualitative data.
- Year-on-Year Growth Trend: We utilize a year-on-year growth trend based on historical market growth and expected future trends. This helps in formulating our growth projections, aligning them with the market’s historical performance.
Research process adopted by TNR involves multiple stages, including data collection, validation, quality checks, and presentation. It’s crucial that the data and information we provide add value to your existing market understanding and expertise. We have also established partnerships with business consulting, research, and survey organizations across regions and globally to collaborate on regional analysis and data validation, ensuring the highest level of accuracy and reliability in our reports.









