Global CBD API Market, By Type, By Dosage Form, By Origin, By Indication, By End User, By Region & Segmental Insights Trends and Forecast, 2024 – 2034
- Industry: Healthcare
- Report ID: TNR-110-1203
- Number of Pages: 420
- Table/Charts : Yes
- July, 2024
- Base Year : 2024
- No. of Companies : 10+
- No. of Countries : 29
- Views : 10135
- Covid Impact Covered: Yes
- War Impact Covered: Yes
- Formats : PDF, Excel, PPT
The CBD API (Cannabidiol Active Pharmaceutical Ingredient) market is witnessing substantial growth driven by increasing consumer awareness and acceptance of CBD-based products for health and wellness. The pharmaceutical, nutraceutical, and cosmetic industries are capitalizing on CBD’s therapeutic properties, such as its anti-inflammatory, analgesic, and anxiolytic effects.
Key trends include the rising demand for natural and plant-based products, advancements in extraction and synthesis technologies, and increased research into CBD’s medical benefits. Opportunities abound in developing innovative delivery methods, expanding product lines, and penetrating emerging markets where regulatory landscapes are becoming more favorable. Companies investing in R&D and forming strategic partnerships are well-positioned to capitalize on these trends. Growth drivers for the CBD API market include expanding legalization and regulatory approvals across various regions, increasing consumer interest in alternative therapies, and growing investment in CBD research and product development. Additionally, the aging global population and rising prevalence of chronic conditions create a robust demand for CBD-based therapeutic solutions, further propelling market expansion.
In Terms of Revenue, the Global CBD API Market was Worth US$ 10.5 Bn in 2023, Anticipated to Witness CAGR of 26.4% During 2024 – 2034.

Trends in the Global CBD API Market
- Advancements in Extraction and Synthesis Technologies: Traditional methods, such as solvent extraction, are being supplemented and replaced by more efficient and environmentally friendly techniques like supercritical CO2 extraction. These advancements enhance the purity and potency of CBD APIs, meeting the stringent quality standards required for pharmaceutical applications. Additionally, the development of synthetic CBD offers a scalable and consistent alternative to plant-derived CBD, addressing supply chain limitations and ensuring a steady supply for manufacturers. This trend is driving increased investment in R&D, as companies strive to improve yield, reduce costs, and produce high-quality CBD APIs that meet regulatory requirements and consumer expectations.
- Expansion of Legalization and Regulatory Approvals: As more countries and regions recognize the therapeutic potential of CBD, they are enacting legislation to legalize its use in various applications, from medical treatments to wellness products. This regulatory shift is opening new markets and increasing the accessibility of CBD APIs for research and commercialization. In regions where CBD was previously restricted, such as parts of Europe, Asia, and South America, new regulations are fostering growth and innovation. Companies that navigate these evolving legal landscapes successfully are poised to benefit from first-mover advantages and can establish a strong market presence in newly opened territories. For instance, in February 2022, Averix Bio submitted a Drug Master File (DMF 036798) to the US Food and Drug Administration (FDA) for the isolation of cannabidiol (CBD). This submission underscores Averix Bio’s commitment to delivering top-quality ingredients for clinical trials, as well as for the pharmaceutical, cosmetic, nutraceutical, and veterinary sectors.
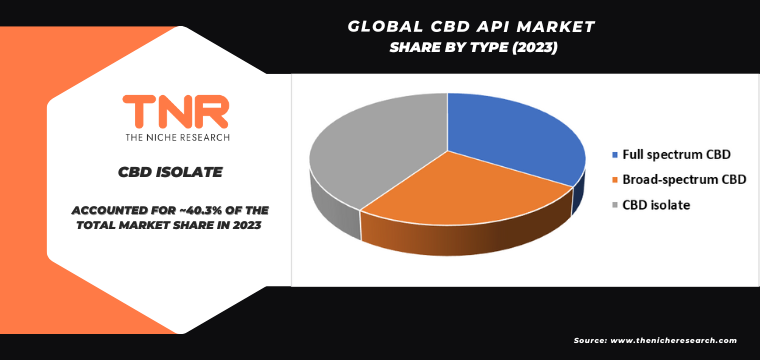
CBD isolate by type category has emerged as a dominant segment in the global CBD API market. This growth is driven by the increasing demand for pure, THC-free CBD products across various industries. Pharmaceutical companies prefer CBD isolate for its consistent potency and lack of psychoactive compounds, making it ideal for formulating precise dosages in medications. In the cosmetic and nutraceutical sectors, the high purity of CBD isolate ensures safety and efficacy in product development. Additionally, consumer preference for non-psychoactive health and wellness products boosts the demand for CBD isolate. The market is further propelled by advancements in extraction technologies that enhance the production efficiency and purity of CBD isolates, meeting the stringent quality standards required by various applications.
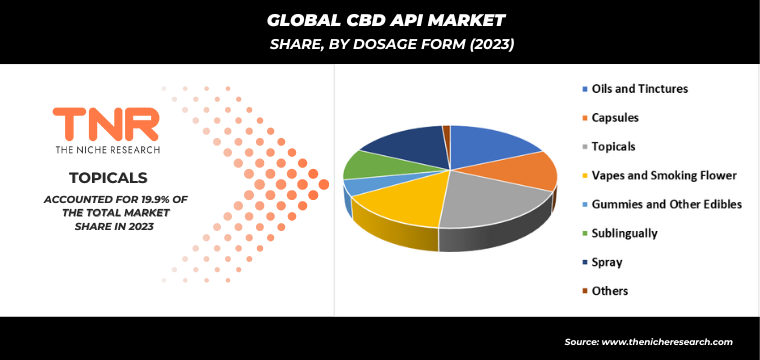
In 2023, oils and tinctures segment solidified its position as the second-largest dosage form category within the global CBD API market. This growth is attributed to the increasing consumer preference for convenient and versatile CBD consumption methods. Oils and tinctures offer precise dosing, rapid absorption, and ease of use, making them popular choices among both new and experienced CBD users. Additionally, their versatility allows for sublingual administration or incorporation into foods and beverages, catering to diverse consumer needs. The segment’s expansion is also fueled by ongoing product innovations, such as enhanced bioavailability and improved flavor profiles. As a result, oils and tinctures continue to capture a significant share of the market, driven by their effectiveness and adaptability.
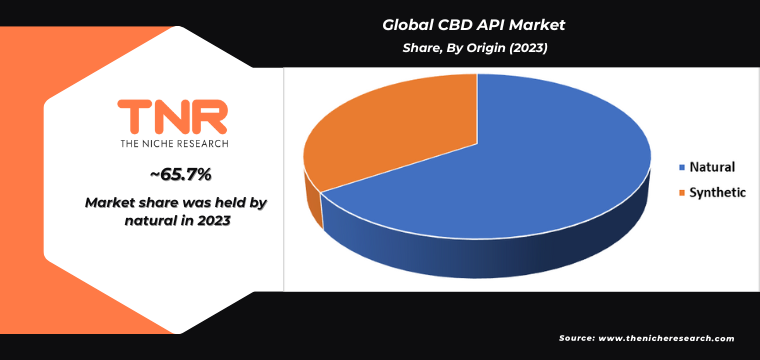
Natural segment, categorized by origin, has exerted significant dominance over the global CBD API market. This prevalence is driven by growing consumer preference for organic and plant-based products, perceived as safer and more effective. Natural CBD APIs are extracted from hemp plants using methods that preserve their purity and potency, meeting the high demand for non-synthetic, clean-label ingredients in pharmaceuticals, nutraceuticals, cosmetics, and veterinary products. Additionally, regulatory bodies increasingly favor natural over synthetic CBD, further boosting this segment. Companies are investing in sustainable cultivation practices and advanced extraction technologies to maintain the quality and consistency of natural CBD APIs. This trend underscores the market’s shift towards environmentally friendly and health-conscious products, solidifying the natural segment’s leading position.
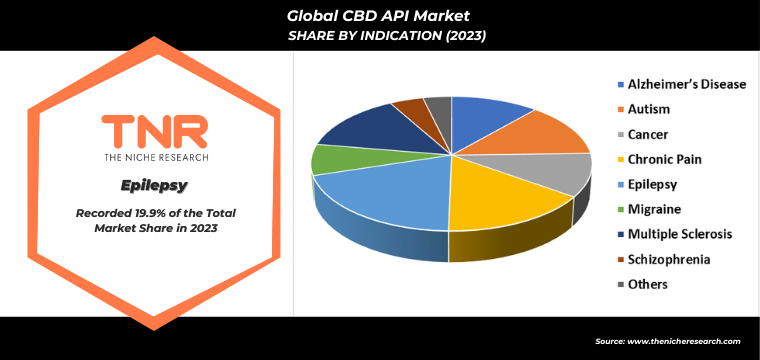
By indication, chronic pain segment is anticipated to grow fastest over the forecast timeline in global CBD API market. This rapid growth is driven by increasing recognition of CBD’s efficacy in managing chronic pain conditions, such as arthritis, neuropathy, and fibromyalgia. Patients and healthcare providers are increasingly turning to CBD as a natural alternative to traditional pain medications, which often come with significant side effects and risk of addiction. Additionally, ongoing research and clinical trials continue to validate CBD’s pain-relieving properties, boosting its acceptance in medical communities. The rising prevalence of chronic pain, particularly among aging populations, further propels demand. This trend underscores the potential of CBD APIs to address unmet needs in pain management, fueling market expansion.
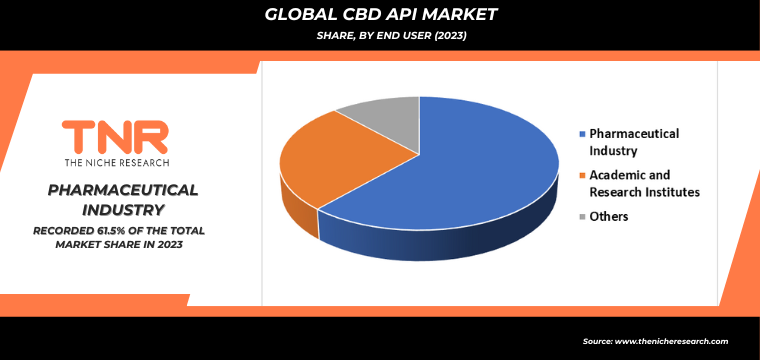
Pharmaceutical industry segment by end user dominated the global CBD API market in 2023 with a CAGR of 7.5%. This growth can be attributed to the increasing acceptance and integration of CBD in medical treatments and therapies. Pharmaceuticals harness CBD’s therapeutic properties to develop medications for a variety of conditions such as epilepsy, chronic pain, and anxiety. The rising demand for high-quality, effective CBD-based products has driven investments and advancements in this sector. Regulatory approvals and ongoing research further bolster the credibility and utilization of CBD in pharmaceuticals, solidifying its leading position in the global CBD API market.
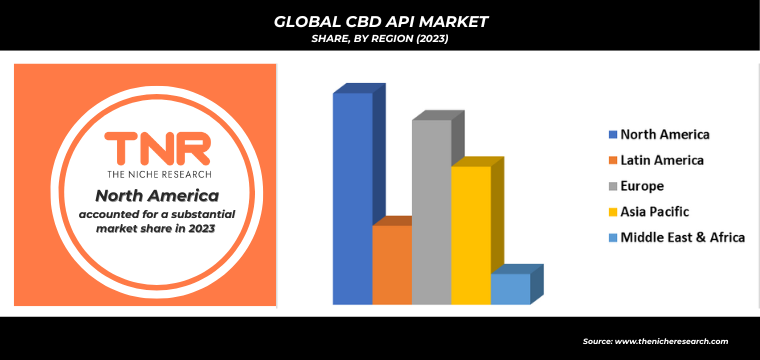
In 2023, North America solidified its dominance in the global CBD API market, contributing a revenue share of 32.9%. This leadership can be attributed to the region’s progressive regulatory environment, increasing consumer awareness, and robust demand for CBD-infused pharmaceutical products. The United States, in particular, has seen substantial investment in CBD research and development, leading to innovative medical applications and therapies. The presence of major industry players and a well-established healthcare infrastructure have facilitated market growth. The growing acceptance of CBD for various therapeutic uses, coupled with favorable legislative developments, has reinforced North America’s prominent position in the global CBD API market.
Competitive Landscape
Some of the players operating in the CBD API Market are
- Averix Bio Llc
- Bedrocan
- Biosyyd Uab
- Biovectra Inc
- Brains
- Cannatrek
- Colombian Golden
- Endopure
- Eurofins
- Gvb Biopharma
- Jordan Process
- Knd Labs
- Purisys
- Recipharm AB
- Vantage Hemp
- Other Industry Participants
Global CBD API Market Scope
| Report Specifications | Details |
| Market Revenue in 2023 | US$ 10.5 Bn |
| Market Size Forecast by 2034 | US$ 137.6 Bn |
| Growth Rate (CAGR) | 26.4% |
| Historic Data | 2016 – 2022 |
| Base Year for Estimation | 2023 |
| Forecast Period | 2024 – 2034 |
| Report Inclusions | Market Size & Estimates, Market Dynamics, Competitive Scenario, Trends, Growth Factors, Market Determinants, Key Investment Segmentation, Product/Service/Solutions Benchmarking |
| Segments Covered | By Type, By Dosage Form, By Origin, By Indication, By End User, By Region |
| Regions Covered | North America, Europe, Asia Pacific, Middle East & Africa, Latin America |
| Countries Covered | U.S., Canada, Mexico, Rest of North America, France, The UK, Spain, Germany, Italy, Nordic Countries (Denmark, Finland, Iceland, Sweden, Norway), Benelux Union (Belgium, The Netherlands, Luxembourg), Rest of Europe, China, Japan, India, New Zealand, Australia, South Korea, Southeast Asia (Indonesia, Thailand, Malaysia, Singapore, Rest of Southeast Asia), Rest of Asia Pacific, Saudi Arabia, UAE, Egypt, Kuwait, South Africa, Rest of Middle East & Africa, Brazil, Argentina, Rest of Latin America |
| Key Players | Averix Bio Llc, Bedrocan, Biosyyd Uab, Biovectra Inc, Brains, Cannatrek, Colombian Golden, Endopure, Eurofins, Gvb Biopharma, Jordan Process, Knd Labs, Purisys , Recipharm AB, Vantage Hemp |
| Customization Scope | Customization allows for the inclusion/modification of content pertaining to geographical regions, countries, and specific market segments. |
| Pricing & Procurement Options | Explore purchase options tailored to your specific research requirements |
| Contact Details | Consult With Our Expert
Japan (Toll-Free): +81 663-386-8111 South Korea (Toll-Free): +82-808- 703-126 Saudi Arabia (Toll-Free): +966 800-850-1643 United Kingdom: +44 753-710-5080 United States: +1 302-232-5106 E-mail: askanexpert@thenicheresearch.com
|
Global CBD API Market
By Type
- Full spectrum CBD
- Broad-spectrum CBD
- CBD isolate
By Dosage form
- Oils and Tinctures
- Capsules
- Topicals
- Balm
- Creams
- Lotion
- Salves
- Vapes and Smoking Flower
- Gummies and Other Edibles
- Sublingually
- Spray
- Others
By Origin
- Natural
- Synthetic
By Indication
- Alzheimer’s Disease
- Autism
- Cancer
- Chronic Pain
- Epilepsy
- Migraine
- Multiple Sclerosis
- Schizophrenia
- Others
By End User
- Pharmaceutical Industry
- Academic and Research Institutes
- Others
By Region
- North America (U.S., Canada, Mexico, Rest of North America)
- Europe (France, The UK, Spain, Germany, Italy, Nordic Countries (Denmark, Finland, Iceland, Sweden, Norway), Benelux Union (Belgium, The Netherlands, Luxembourg), Rest of Europe)
- Asia Pacific (China, Japan, India, New Zealand, Australia, South Korea, Southeast Asia (Indonesia, Thailand, Malaysia, Singapore, Rest of Southeast Asia), Rest of Asia Pacific)
- Middle East & Africa (Saudi Arabia, UAE, Egypt, Kuwait, South Africa, Rest of Middle East & Africa)
- Latin America (Brazil, Argentina, Rest of Latin America)
Report Layout:
Table of Contents
Note: This ToC is tentative and can be changed according to the research study conducted during the course of report completion.
**Exclusive for Multi-User and Enterprise User.
Global CBD API Market
By Type
- Full spectrum CBD
- Broad-spectrum CBD
- CBD isolate
By Dosage form
- Oils and Tinctures
- Capsules
- Topicals
- Balm
- Creams
- Lotion
- Salves
- Vapes and Smoking Flower
- Gummies and Other Edibles
- Sublingually
- Spray
- Others
By Origin
- Natural
- Synthetic
By Indication
- Alzheimer’s Disease
- Autism
- Cancer
- Chronic Pain
- Epilepsy
- Migraine
- Multiple Sclerosis
- Schizophrenia
- Others
By End User
- Pharmaceutical Industry
- Academic and Research Institutes
- Others
By Region
- North America (U.S., Canada, Mexico, Rest of North America)
- Europe (France, The UK, Spain, Germany, Italy, Nordic Countries (Denmark, Finland, Iceland, Sweden, Norway), Benelux Union (Belgium, The Netherlands, Luxembourg), Rest of Europe)
- Asia Pacific (China, Japan, India, New Zealand, Australia, South Korea, Southeast Asia (Indonesia, Thailand, Malaysia, Singapore, Rest of Southeast Asia), Rest of Asia Pacific)
- Middle East & Africa (Saudi Arabia, UAE, Egypt, Kuwait, South Africa, Rest of Middle East & Africa)
- Latin America (Brazil, Argentina, Rest of Latin America)
The Niche Research approach encompasses both primary and secondary research methods to provide comprehensive insights. While primary research is the cornerstone of our studies, we also incorporate secondary research sources such as company annual reports, premium industry databases, press releases, industry journals, and white papers.
Within our primary research, we actively engage with various industry stakeholders, conducting paid interviews and surveys. Our meticulous analysis extends to every market participant in major countries, allowing us to thoroughly examine their portfolios, calculate market shares, and segment revenues.
Our data collection primarily focuses on individual countries within our research scope, enabling us to estimate regional market sizes. Typically, we employ a bottom-up approach, meticulously tracking trends in different countries. We analyze growth drivers, constraints, technological innovations, and opportunities for each country, ultimately arriving at regional figures.Our process begins by examining the growth prospects of each country. Building upon these insights, we project growth and trends for the entire region. Finally, we utilize our proprietary model to refine estimations and forecasts.
Our data validation standards are integral to ensuring the reliability and accuracy of our research findings. Here’s a breakdown of our data validation processes and the stakeholders we engage with during our primary research:
- Supply Side Analysis: We initiate a supply side analysis by directly contacting market participants, through telephonic interviews and questionnaires containing both open-ended and close-ended questions. We gather information on their portfolios, segment revenues, developments, and growth strategies.
- Demand Side Analysis: To gain insights into adoption trends and consumer preferences, we reach out to target customers and users (non-vendors). This information forms a vital part of the qualitative analysis section of our reports, covering market dynamics, adoption trends, consumer behavior, spending patterns, and other related aspects.
- Consultant Insights: We tap into the expertise of our partner consultants from around the world to obtain their unique viewpoints and perspectives. Their insights contribute to a well-rounded understanding of the markets under investigation.
- In-House Validation: To ensure data accuracy and reliability, we conduct cross-validation of data points and information through our in-house team of consultants and utilize advanced data modeling tools for thorough verification.
The forecasts we provide are based on a comprehensive assessment of various factors, including:
- Market Trends and Past Performance (Last Five Years): We accurately analyze market trends and performance data from preceding five years to identify historical patterns and understand the market’s evolution.
- Historical Performance and Growth of Market Participants: We assess the historical performance and growth trajectories of key market participants. This analysis provides insights into the competitive landscape and individual company strategies.
- Market Determinants Impact Analysis (Next Eight Years): We conduct a rigorous analysis of the factors that are projected to influence the market over the next eight years. This includes assessing both internal and external determinants that can shape market dynamics.
- Drivers and Challenges for the Forecast Period:Identify the factors expected to drive market growth during the forecast period, as well as the challenges that the industry may face. This analysis aids in deriving an accurate growth rate projection.
- New Acquisitions, Collaborations, or Partnerships: We keep a close watch on any new acquisitions, collaborations, or partnerships within the industry. These developments can have a significant impact on market dynamics and competitiveness.
- Macro and Micro Factors Analysis:A thorough examination of both macro-level factors (e.g., economic trends, regulatory changes) and micro-level factors (e.g., technological advancements, consumer preferences) that may influence the market during the forecast period.
- End-User Sentiment Analysis: To understand the market from the end-user perspective, we conduct sentiment analysis. This involves assessing the sentiment, preferences, and feedback of the end-users, which can provide valuable insights into market trends.
- Perspective of Primary Participants: Insights gathered directly from primary research participants play a crucial role in shaping our forecasts. Their perspectives and experiences provide valuable qualitative data.
- Year-on-Year Growth Trend: We utilize a year-on-year growth trend based on historical market growth and expected future trends. This helps in formulating our growth projections, aligning them with the market’s historical performance.
Research process adopted by TNR involves multiple stages, including data collection, validation, quality checks, and presentation. It’s crucial that the data and information we provide add value to your existing market understanding and expertise. We have also established partnerships with business consulting, research, and survey organizations across regions and globally to collaborate on regional analysis and data validation, ensuring the highest level of accuracy and reliability in our reports.









