Global Cerebral Embolic Protection Devices for TAVI Market: By Device Type, By End User, By Region & Segmental Insights Trends and Forecast, 2024 – 2034
- Industry: Healthcare
- Report ID: TNR-110-1269
- Number of Pages: 420
- Table/Charts : Yes
- August, 2024
- Base Year : 2024
- No. of Companies : 10+
- No. of Countries : 29
- Views : 10048
- Covid Impact Covered: Yes
- War Impact Covered: Yes
- Formats : PDF, Excel, PPT
Cerebral embolic protection (CEP) devices are specialized medical tools used during transcatheter aortic valve implantation (TAVI), a minimally invasive procedure to replace the aortic valve in patients with aortic stenosis. These devices are designed to capture and remove embolic debris that can dislodge during TAVI and potentially travel to the brain, causing strokes or other neurological complications. The use of CEP devices in TAVI is essential because the procedure involves manipulating the calcified and diseased aortic valve, which can release debris into the bloodstream. Without protection, this debris can reach the cerebral circulation, leading to embolic strokes. Strokes are a significant concern in TAVI procedures, particularly in high-risk patients, as they can result in long-term disability or even death.
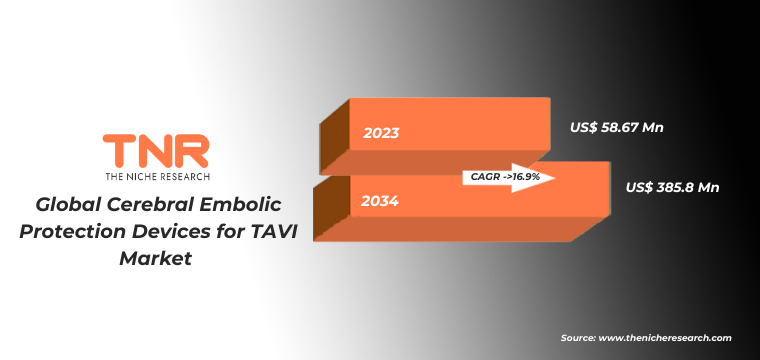
The need for CEP devices has become more evident as TAVI procedures have expanded to include a broader range of patients, including those at intermediate and low surgical risk. As TAVI becomes more common, the adoption of CEP devices is expected to grow, driven by the increasing awareness of their benefits in reducing stroke risk and enhancing the overall safety of the procedure. The global cerebral embolic protection devices for TAVI market was worth USD 58.67 Mn in 2023 and is expected to register a CAGR of 16.9% during the forecast period (2024 – 2034). Factors such as growing prevalence of cardiovascular diseases specifically aortic stenosis, increasing government fundings to carry out R&D activities, supportive regulations and quick approvals are propelling market growth.
For instance, cardiovascular disease remains the leading cause of mortality in the United States. According to the latest data from the American Heart Association (AHA) for 2024, cardiovascular disease causes 2,552 deaths daily, with coronary heart disease (CHD) being the most significant contributor. Following CHD, stroke accounts for 17.3%, other forms of CVD for 16.8%, high blood pressure for 12.9%, heart failure for 9.2%, and arterial diseases for 2.6%. As of 2024, heart disease continues to be a critical concern, with approximately 1,905 daily deaths related to heart conditions, including heart attacks. Additionally, someone in the U.S. suffers a heart attack every 40 seconds. Stroke is also a major issue, with 446 daily deaths and a stroke occurring approximately every 3 minutes and 14 seconds.
Individuals have started preferring minimally invasive therapeutic procedures. According to the National Center for Biotechnology Information, patients find minimally invasive therapeutic treatments more appealing due to their reduced need for incisions, decreased discomfort, absence of visible scars, shorter hospital stays, and consequent cost savings. Furthermore, surgeons reportedly favor these procedures because they expedite the healing process and reduce the risk of complications. Various product approvals and continuous investments by private companies and government authorities are fostering R&D activities, which is also contributing to the growth of the market. For instance, on 9 May 2023, Emboline, Inc. disclosed the initiation of the inaugural patient’s treatment in the Protect the Head-to-Head clinical trial, conducted under an investigational device exemption (IDE), at New York-Presbyterian Hospital/Columbia University Medical Center in New York, NY. The Emboliner represents the pioneering device offering comprehensive protection for both the brain and the body against ischemic events, including strokes resulting from embolic debris released into the bloodstream during transcatheter heart procedures.
Market Challenges: Global Cerebral Embolic Protection Devices for TAVI Market
- Cost Concerns: The average cost of a CEP device ranges from $2,500 to $4,000, adding a significant expense to TAVI procedures, particularly in regions with limited healthcare funding. In emerging markets, where healthcare budgets are tighter, this cost factor limits broader adoption, affecting market penetration.
- Limited Adoption in Some Regions: In regions like Latin America and parts of Asia-Pacific, the adoption of CEP devices remains below 20% of all TAVI procedures due to a lack of awareness and training. Moreover, logistical challenges in integrating CEP technology into the procedural workflow further hinder its adoption.
- Competition from Alternative Therapies: Alternatives to CEP devices, such as pharmacological interventions, continue to pose a challenge. However, clinical data favoring CEP devices, showing a 30-35% higher efficacy in preventing strokes, are helping to mitigate this competition.
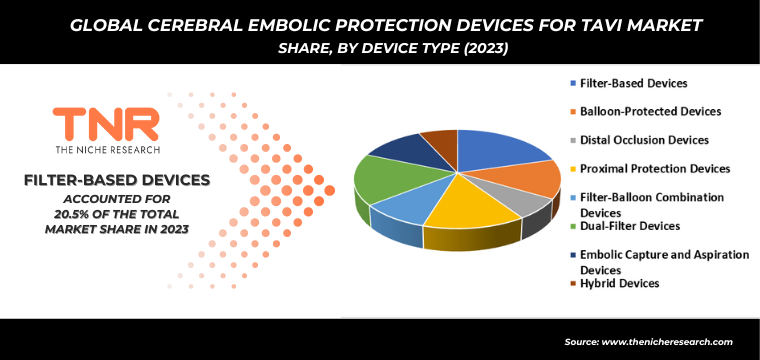
Filter-Based Devices are the most widely used among the listed cerebral embolic protection devices in TAVI market procedures. Their dominance is due to their proven efficacy in capturing and preventing embolic material from reaching the brain, reducing the risk of stroke. These devices have been extensively validated in clinical trials and real-world settings, leading to widespread adoption. Their design allows for effective filtering of debris without significant disruption to blood flow, making them the preferred choice among healthcare providers for ensuring patient safety during TAVI. For instance:
- Sentinel Cerebral Protection System by Claret Medical (acquired by Boston Scientific) is one of the most prominent filter-based devices. It has been used in over 40% of TAVI procedures in the United States and has shown a 63% reduction in new ischemic brain lesions post-TAVI, according to clinical studies.
- Keystone Heart’s TriGuard 3 uses a filter-based approach with a coverage rate of nearly 90% of the cerebral vasculature during TAVI, minimizing the risk of embolic events. The device has shown a 45% reduction in clinically apparent strokes in various studies.
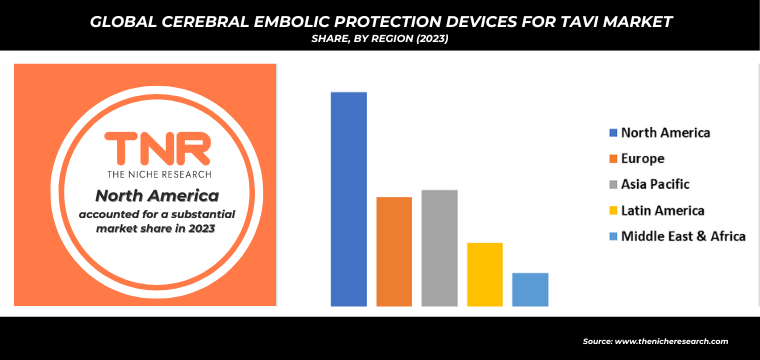
In North America, particularly in the United States, the cerebral embolic protection devices for TAVI market leads globally, capturing approximately 45% of total sales. This dominance is driven by the U.S.’s high volume of TAVI procedures, with over 100,000 performed annually as of 2023. The well-established healthcare system in the U.S. supports widespread adoption of CEP devices, facilitated by favorable reimbursement policies and a robust infrastructure that enables extensive use of advanced medical technologies. Additionally, key players like Boston Scientific and Edwards Lifesciences have a strong presence in the region, contributing to the market’s growth and ensuring that the latest CEP innovations are readily available. This combination of procedural volume, healthcare support, and leading technology adoption underpins North America’s significant share of the global cerebral embolic protection devices for TAVI market.
Competitive Insights: Global Cerebral Embolic Protection Devices for TAVI Market
The cerebral embolic protection devices for TAVI market is highly competitive, with several key players leading innovation and market expansion. Start-ups are contributing to 15% of market innovations, focusing on niche markets and bringing new technologies that could disrupt existing market dynamics. A few of the key companies operating in the market include:
- Allium Medical Inc.
- Boston Scientific Corporation
- CardioGard, Ltd
- Claret Medical, Inc
- Edwards Lifesciences Corporation
- Emboline, Inc.
- Keystone Heart, A Venus Medtech Company
- Medtronic
- Sentinel Cerebral Protection System
- Other Market Participants
Global Cerebral Embolic Protection Devices for TAVI Market Scope:
| Report Specifications | Details |
| Market Revenue in 2023 | US$ 58.67 Mn |
| Market Size Forecast by 2034 | US$ 385.8 Mn |
| Growth Rate (CAGR) | 16.9% |
| Historic Data | 2016 – 2022 |
| Base Year for Estimation | 2023 |
| Forecast Period | 2024 – 2034 |
| Report Inclusions | Market Size & Estimates, Market Dynamics, Competitive Scenario, Trends, Growth Factors, Market Determinants, Key Investment Segmentation, Product/Service/Solutions Benchmarking |
| Segments Covered | By Device Type, By End User, By Region |
| Regions Covered | North America, Europe, Asia Pacific, Middle East & Africa, Latin America |
| Countries Covered | U.S., Canada, Mexico, Rest of North America, France, The UK, Spain, Germany, Italy, Nordic Countries (Denmark, Finland, Iceland, Sweden, Norway), Benelux Union (Belgium, The Netherlands, Luxembourg), Rest of Europe, China, Japan, India, New Zealand, Australia, South Korea, Southeast Asia (Indonesia, Thailand, Malaysia, Singapore, Rest of Southeast Asia), Rest of Asia Pacific, Saudi Arabia, UAE, Egypt, Kuwait, South Africa, Rest of Middle East & Africa, Brazil, Argentina, Rest of Latin America |
| Key Players | Allium Medical Inc., Boston Scientific Corporation, CardioGard, Ltd, Claret Medical, Inc, Edwards Lifesciences Corporation, Emboline, Inc., Keystone Heart, A Venus Medtech Company, Medtronic, Sentinel Cerebral Protection System |
| Customization Scope | Customization allows for the inclusion/modification of content pertaining to geographical regions, countries, and specific market segments. |
| Pricing & Procurement Options | Explore purchase options tailored to your specific research requirements |
| Contact Details | Consult With Our Expert
Japan (Toll-Free): +81 663-386-8111 South Korea (Toll-Free): +82-808- 703-126 Saudi Arabia (Toll-Free): +966 800-850-1643 United Kingdom: +44 753-710-5080 United States: +1 302-232-5106 E-mail: askanexpert@thenicheresearch.com
|
Global Cerebral Embolic Protection Devices for TAVI Market
By Device Type
- Filter-Based Devices
- Balloon-Protected Devices
- Distal Occlusion Devices
- Proximal Protection Devices
- Filter-Balloon Combination Devices
- Dual-Filter Devices
- Embolic Capture and Aspiration Devices
- Hybrid Devices
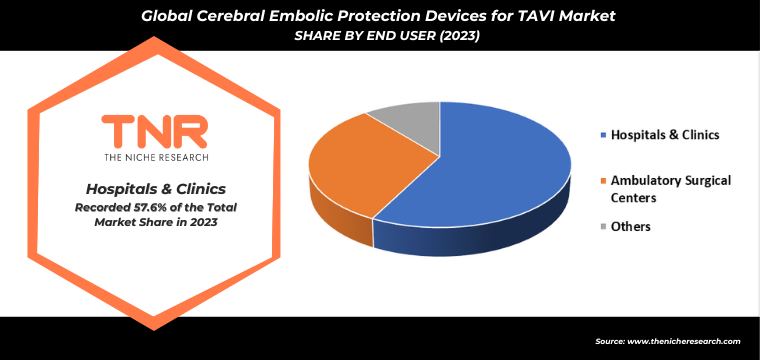
By End User
- Hospitals & Clinics
- Ambulatory Surgical Centers
- Others
By Region
- North America (U.S., Canada, Mexico, Rest of North America)
- Europe (France, The UK, Spain, Germany, Italy, Nordic Countries (Denmark, Finland, Iceland, Sweden, Norway), Benelux Union (Belgium, The Netherlands, Luxembourg), Rest of Europe)
- Asia Pacific (China, Japan, India, New Zealand, Australia, South Korea, Southeast Asia (Indonesia, Thailand, Malaysia, Singapore, Rest of Southeast Asia), Rest of Asia Pacific)
- Middle East & Africa (Saudi Arabia, UAE, Egypt, Kuwait, South Africa, Rest of Middle East & Africa)
- Latin America (Brazil, Argentina, Rest of Latin America)
Report Layout:
Table of Contents
Note: This ToC is tentative and can be changed according to the research study conducted during the course of report completion.
**Exclusive for Multi-User and Enterprise User.
Global Cerebral Embolic Protection Devices for TAVI Market
By Device Type
- Filter-Based Devices
- Balloon-Protected Devices
- Distal Occlusion Devices
- Proximal Protection Devices
- Filter-Balloon Combination Devices
- Dual-Filter Devices
- Embolic Capture and Aspiration Devices
- Hybrid Devices
By End User
- Hospitals & Clinics
- Ambulatory Surgical Centers
- Others
By Region
- North America (U.S., Canada, Mexico, Rest of North America)
- Europe (France, The UK, Spain, Germany, Italy, Nordic Countries (Denmark, Finland, Iceland, Sweden, Norway), Benelux Union (Belgium, The Netherlands, Luxembourg), Rest of Europe)
- Asia Pacific (China, Japan, India, New Zealand, Australia, South Korea, Southeast Asia (Indonesia, Thailand, Malaysia, Singapore, Rest of Southeast Asia), Rest of Asia Pacific)
- Middle East & Africa (Saudi Arabia, UAE, Egypt, Kuwait, South Africa, Rest of Middle East & Africa)
- Latin America (Brazil, Argentina, Rest of Latin America)
The Niche Research approach encompasses both primary and secondary research methods to provide comprehensive insights. While primary research is the cornerstone of our studies, we also incorporate secondary research sources such as company annual reports, premium industry databases, press releases, industry journals, and white papers.
Within our primary research, we actively engage with various industry stakeholders, conducting paid interviews and surveys. Our meticulous analysis extends to every market participant in major countries, allowing us to thoroughly examine their portfolios, calculate market shares, and segment revenues.
Our data collection primarily focuses on individual countries within our research scope, enabling us to estimate regional market sizes. Typically, we employ a bottom-up approach, meticulously tracking trends in different countries. We analyze growth drivers, constraints, technological innovations, and opportunities for each country, ultimately arriving at regional figures.Our process begins by examining the growth prospects of each country. Building upon these insights, we project growth and trends for the entire region. Finally, we utilize our proprietary model to refine estimations and forecasts.
Our data validation standards are integral to ensuring the reliability and accuracy of our research findings. Here’s a breakdown of our data validation processes and the stakeholders we engage with during our primary research:
- Supply Side Analysis: We initiate a supply side analysis by directly contacting market participants, through telephonic interviews and questionnaires containing both open-ended and close-ended questions. We gather information on their portfolios, segment revenues, developments, and growth strategies.
- Demand Side Analysis: To gain insights into adoption trends and consumer preferences, we reach out to target customers and users (non-vendors). This information forms a vital part of the qualitative analysis section of our reports, covering market dynamics, adoption trends, consumer behavior, spending patterns, and other related aspects.
- Consultant Insights: We tap into the expertise of our partner consultants from around the world to obtain their unique viewpoints and perspectives. Their insights contribute to a well-rounded understanding of the markets under investigation.
- In-House Validation: To ensure data accuracy and reliability, we conduct cross-validation of data points and information through our in-house team of consultants and utilize advanced data modeling tools for thorough verification.
The forecasts we provide are based on a comprehensive assessment of various factors, including:
- Market Trends and Past Performance (Last Five Years): We accurately analyze market trends and performance data from preceding five years to identify historical patterns and understand the market’s evolution.
- Historical Performance and Growth of Market Participants: We assess the historical performance and growth trajectories of key market participants. This analysis provides insights into the competitive landscape and individual company strategies.
- Market Determinants Impact Analysis (Next Eight Years): We conduct a rigorous analysis of the factors that are projected to influence the market over the next eight years. This includes assessing both internal and external determinants that can shape market dynamics.
- Drivers and Challenges for the Forecast Period:Identify the factors expected to drive market growth during the forecast period, as well as the challenges that the industry may face. This analysis aids in deriving an accurate growth rate projection.
- New Acquisitions, Collaborations, or Partnerships: We keep a close watch on any new acquisitions, collaborations, or partnerships within the industry. These developments can have a significant impact on market dynamics and competitiveness.
- Macro and Micro Factors Analysis:A thorough examination of both macro-level factors (e.g., economic trends, regulatory changes) and micro-level factors (e.g., technological advancements, consumer preferences) that may influence the market during the forecast period.
- End-User Sentiment Analysis: To understand the market from the end-user perspective, we conduct sentiment analysis. This involves assessing the sentiment, preferences, and feedback of the end-users, which can provide valuable insights into market trends.
- Perspective of Primary Participants: Insights gathered directly from primary research participants play a crucial role in shaping our forecasts. Their perspectives and experiences provide valuable qualitative data.
- Year-on-Year Growth Trend: We utilize a year-on-year growth trend based on historical market growth and expected future trends. This helps in formulating our growth projections, aligning them with the market’s historical performance.
Research process adopted by TNR involves multiple stages, including data collection, validation, quality checks, and presentation. It’s crucial that the data and information we provide add value to your existing market understanding and expertise. We have also established partnerships with business consulting, research, and survey organizations across regions and globally to collaborate on regional analysis and data validation, ensuring the highest level of accuracy and reliability in our reports.









