Global Clinical Trial Management System Market: By Component, By Deployment Model, By Organization Size, By Application, By End User, By Region & Segmental Insights Trends and Forecast, 2024 – 2034
- Industry: Healthcare
- Report ID: TNR-110-1182
- Number of Pages: 420
- Table/Charts : Yes
- June, 2024
- Base Year : 2024
- No. of Companies : 10+
- No. of Countries : 29
- Views : 10196
- Covid Impact Covered: Yes
- War Impact Covered: Yes
- Formats : PDF, Excel, PPT
A clinical trial management system (CTMS) is a software platform designed to streamline the planning, tracking, and management of clinical trials in pharmaceutical, biotechnology, and medical device industries. CTMS facilitates the operational aspects of trials by integrating functions such as study protocol management, patient enrolment, scheduling, data collection, and reporting. It enables stakeholders including sponsors, investigators, and contract research organizations (CROs) to collaborate effectively, ensuring trials are conducted efficiently and in compliance with regulatory requirements.
CTMS platforms provide real-time insights into trial progress, patient recruitment metrics, and data quality, allowing for proactive decision-making to mitigate risks and optimize resource allocation. As clinical trials become more complex and globalized, CTMS plays a crucial role in enhancing trial efficiency, reducing costs, and accelerating the development of new therapies and treatments through streamlined trial management processes.
The primary demand driver for clinical trial management systems (CTMS) is the increasing complexity and globalization of clinical trials. As trials expand to include larger and more diverse participant pools across multiple geographic regions, the need for efficient and centralized management becomes paramount. CTMS platforms offer comprehensive tools for protocol management, patient recruitment, data collection, and regulatory compliance, thereby optimizing trial operations and ensuring consistency in data handling and reporting. Another significant driver is the stringent regulatory environment governing clinical research, where CTMS facilitates adherence to protocols and regulatory requirements, ensuring data integrity and patient safety throughout the trial lifecycle.
Moreover, the adoption of digital solutions in healthcare continues to propel the demand for CTMS, as pharmaceutical companies and contract research organizations (CROs) seek to enhance trial efficiency, reduce timelines, and control costs through streamlined processes and real-time data insights. These factors collectively underscore the critical role of CTMS in driving efficiency and compliance in modern clinical research endeavours globally.
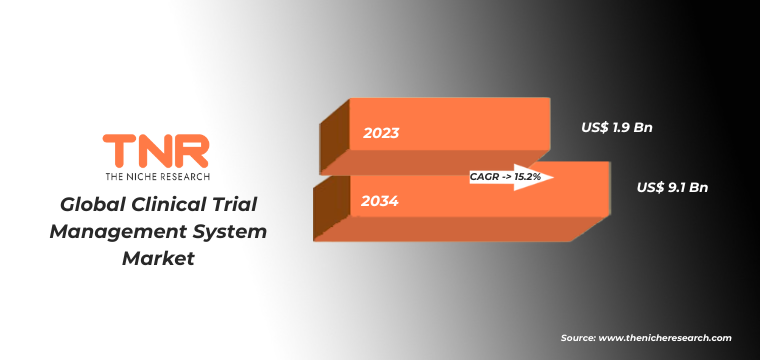
In terms of revenue, the global clinical trial management system market was worth US$ 1.9 Bn in 2023, anticipated to witness CAGR of 15.2% during 2024 – 2034.
Global Clinical Trial Management System Market Dynamics
Increasing Complexity of Clinical Trials: The rise in complexity due to larger study populations, diverse geographic locations, and intricate study designs necessitates robust CTMS solutions. These systems streamline trial operations, ensuring compliance with regulatory standards and enhancing data quality through integrated functionalities like electronic data capture (EDC) and real-time analytics.
Regulatory Stringency: Stringent regulatory requirements globally drive the adoption of CTMS to ensure adherence to protocols, data integrity, and patient safety throughout clinical trials. CTMS platforms facilitate documentation and reporting processes, supporting regulatory submissions and audits.
Technological Advancements: Continuous innovation in CTMS technologies, such as cloud-based solutions and mobile applications, improves accessibility, scalability, and interoperability. These advancements enhance collaboration among trial stakeholders and enable real-time data management and analysis.
Service Segment is Projected as the Fastest Growing Segment in the Global Clinical Trial Management System Market During the Forecast Period (2024 – 2034).
Services associated with clinical trial management systems (CTMS) are crucial demand drivers, offering essential support for efficient trial execution and compliance. Providers offer a range of services including implementation, training, support, and maintenance of CTMS platforms, ensuring they meet the specific needs of pharmaceutical companies, biotech firms, and contract research organizations (CROs). Implementation services involve customizing CTMS to align with trial protocols and regulatory requirements, optimizing workflows for seamless data management and reporting. Training services ensure that trial personnel are proficient in using the system, enhancing user adoption and operational efficiency.
Ongoing support services offer technical assistance and troubleshooting, critical for maintaining uninterrupted trial operations. Additionally, maintenance services ensure CTMS platforms remain up-to-date with regulatory changes and technological advancements, minimizing downtime and enhancing system reliability. The demand for these services is driven by the increasing complexity of clinical trials, stringent regulatory requirements, and the growing preference for outsourcing non-core activities to specialized providers. As clinical research continues to expand globally, these services play a pivotal role in enabling efficient, compliant, and cost-effective trial management.
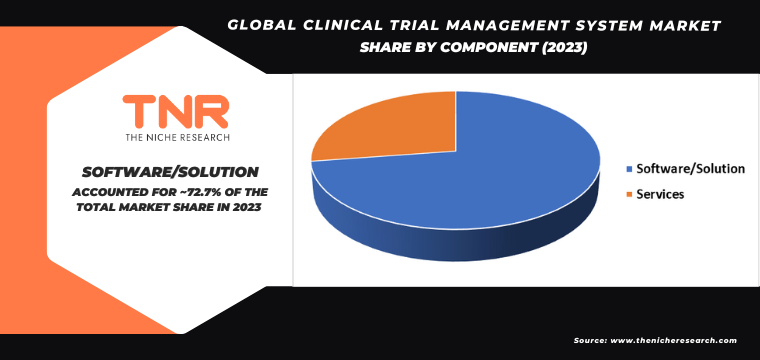
By End User Contact Research Organization Segment had the Highest Share in the Global Clinical Trial Management System Market in 2023.
Contract research organizations (CROs) drive significant demand for Clinical Trial Management Systems (CTMS) due to their pivotal role in managing clinical trials on behalf of pharmaceutical, biotechnology, and medical device companies. CTMS enables CROs to efficiently oversee all phases of clinical trials, from study planning and protocol development to site management, patient recruitment, and data collection. These systems enhance operational efficiency by automating tasks, ensuring compliance with regulatory requirements, and providing real-time insights into trial progress and data quality.
As CROs manage multiple trials simultaneously across global locations, integrated CTMS platforms streamline communication and data integration among various stakeholders, including sponsors, investigators, and regulatory authorities. Moreover, the increasing complexity of clinical trials, coupled with the growing trend towards outsourcing clinical research activities to CROs, drives the adoption of advanced CTMS solutions. By leveraging CTMS capabilities, CROs can optimize resource allocation, minimize trial timelines, and enhance overall trial success rates, thereby meeting the evolving demands of sponsors for efficient and cost-effective clinical trial management solutions.
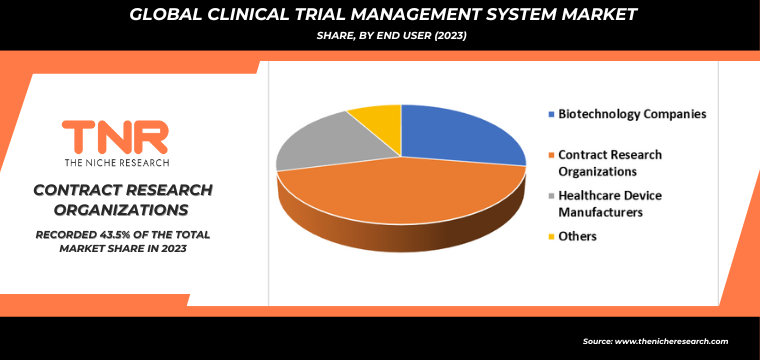
Europe is Projected as One of the Fastest Growing Regions in the Clinical Trial Management System Market.
In Europe, the demand for Clinical Trial Management Systems (CTMS) is driven by several key factors that enhance clinical research efficiency and compliance with regulatory standards. The region’s stringent regulatory environment, governed by bodies like the European Medicines Agency (EMA), necessitates robust CTMS solutions to streamline the complex processes involved in conducting clinical trials. These systems provide comprehensive tools for planning, tracking, and managing all aspects of trials, from patient recruitment and data collection to regulatory reporting and compliance documentation.
Additionally, the increasing globalization of clinical trials and the adoption of electronic data capture (EDC) systems amplify the need for integrated CTMS platforms that can facilitate seamless data management and collaboration across multiple sites and countries. Moreover, the emphasis on patient safety, data integrity, and operational efficiency further underscores the importance of CTMS in ensuring clinical trial success and accelerating the development of new therapies and treatments within the European healthcare landscape. Furthermore, the rise of contract research organizations (CROs) and biopharmaceutical companies outsourcing clinical trials to optimize costs and accelerate drug development timelines contributes significantly to market expansion.
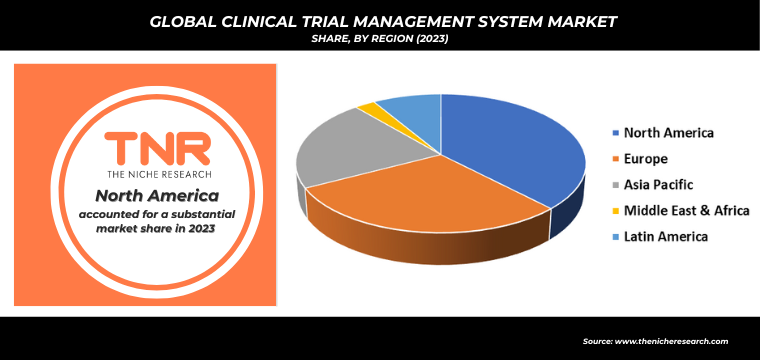
Competitive Landscape: Global Clinical Trial Management System Market:
- Calyx
- Clario
- Clinion
- DATATRAK International, Inc.
- IQVIA Inc.
- Labcorp
- Medidata Solutions, Inc.
- Oracle
- PHARMASEAL
- RealTime
- SimpleTrials
- Veeva Systems
- Wipro Limited
- Other Market Participants
Global Clinical Trial Management System Market Scope
| Report Specifications | Details |
| Market Revenue in 2023 | US$ 1.9 Bn |
| Market Size Forecast by 2034 | US$ 9.1 Bn |
| Growth Rate (CAGR) | 15.2% |
| Historic Data | 2016 – 2022 |
| Base Year for Estimation | 2023 |
| Forecast Period | 2024 – 2034 |
| Report Inclusions | Market Size & Estimates, Market Dynamics, Competitive Scenario, Trends, Growth Factors, Market Determinants, Key Investment Segmentation, Product/Service/Solutions Benchmarking |
| Segments Covered | By Component, By Deployment Model, By Organization Size, By Application, By End User, By Region |
| Regions Covered | North America, Europe, Asia Pacific, Middle East & Africa, Latin America |
| Countries Covered | U.S., Canada, Mexico, Rest of North America, France, The UK, Spain, Germany, Italy, Nordic Countries (Denmark, Finland, Iceland, Sweden, Norway), Benelux Union (Belgium, The Netherlands, Luxembourg), Rest of Europe, China, Japan, India, New Zealand, Australia, South Korea, Southeast Asia (Indonesia, Thailand, Malaysia, Singapore, Rest of Southeast Asia), Rest of Asia Pacific, Saudi Arabia, UAE, Egypt, Kuwait, South Africa, Rest of Middle East & Africa, Brazil, Argentina, Rest of Latin America |
| Key Players | Calyx, Clario, Clinion, DATATRAK International, Inc., IQVIA Inc., Labcorp, Medidata Solutions, Inc., Oracle, PHARMASEAL, RealTime, SimpleTrials, Veeva Systems, Wipro Limited |
| Customization Scope | Customization allows for the inclusion/modification of content pertaining to geographical regions, countries, and specific market segments. |
| Pricing & Procurement Options | Explore purchase options tailored to your specific research requirements |
| Contact Details | Consult With Our Expert
Japan (Toll-Free): +81 663-386-8111 South Korea (Toll-Free): +82-808- 703-126 Saudi Arabia (Toll-Free): +966 800-850-1643 United Kingdom: +44 753-710-5080 United States: +1 302-232-5106 E-mail: askanexpert@thenicheresearch.com
|
Global Clinical Trial Management System Market
By Component
- Software/Solution
- Early Phase
- Phase I
- Phase II-III
- Late Phase Clinical Research
- Others
- Services
- Consultancy & Advisory
- Software & Data Migration
- Employee Training
- Software Customization
- Software Redesign
- Clinical Trial Software Implementation
- Others
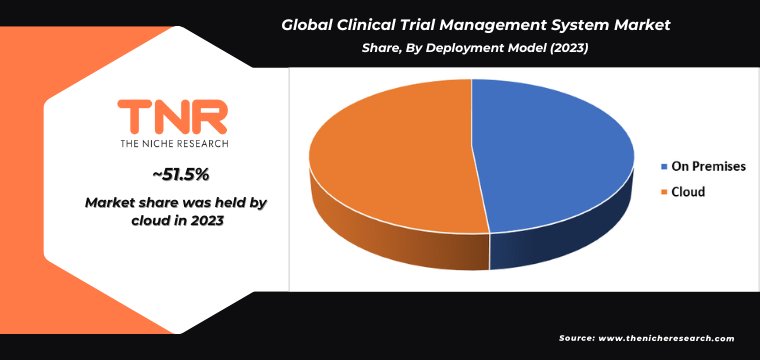
By Deployment Model
- On Premises
- Cloud
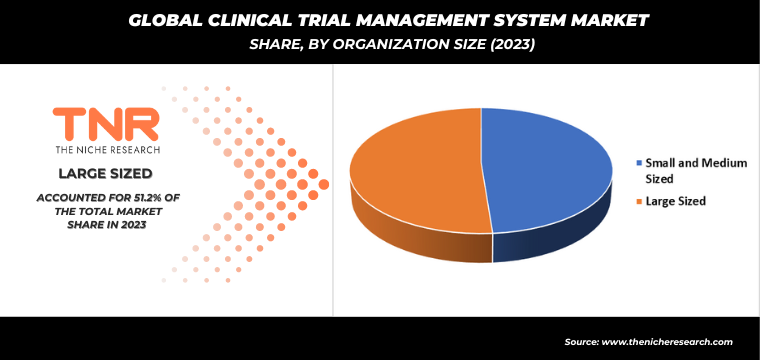
By Organization Size
- Small and Medium Sized
- Large Sized
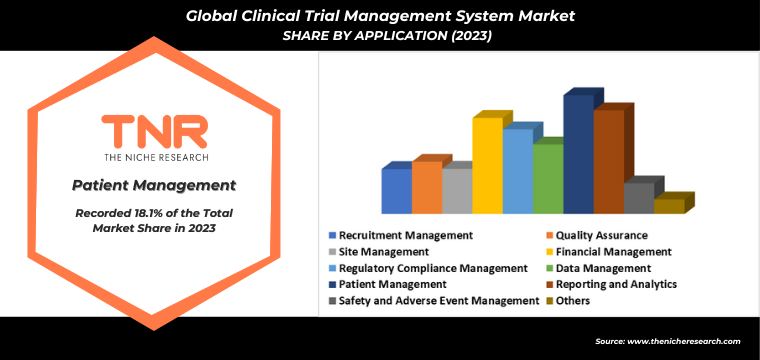
By Application
- Recruitment Management
- Quality Assurance
- Site Management
- Financial Management
- Regulatory Compliance Management
- Data Management
- Patient Management
- Reporting and Analytics
- Safety and Adverse Event Management
- Others
By End User
- Biotechnology Companies
- Contract Research Organizations
- Healthcare Device Manufacturers
- Others
By Region
- North America (U.S., Canada, Mexico, Rest of North America)
- Europe (France, The UK, Spain, Germany, Italy, Nordic Countries (Denmark, Finland, Iceland, Sweden, Norway), Benelux Union (Belgium, The Netherlands, Luxembourg), Rest of Europe)
- Asia Pacific (China, Japan, India, New Zealand, Australia, South Korea, Southeast Asia (Indonesia, Thailand, Malaysia, Singapore, Rest of Southeast Asia), Rest of Asia Pacific)
- Middle East & Africa (Saudi Arabia, UAE, Egypt, Kuwait, South Africa, Rest of Middle East & Africa)
- Latin America (Brazil, Argentina, Rest of Latin America)
Report Layout:
Table of Contents
Note: This ToC is tentative and can be changed according to the research study conducted during the course of report completion.
**Exclusive for Multi-User and Enterprise User.
Global Clinical Trial Management System Market
By Component
- Software/Solution
- Early Phase
- Phase I
- Phase II-III
- Late Phase Clinical Research
- Others
- Services
- Consultancy & Advisory
- Software & Data Migration
- Employee Training
- Software Customization
- Software Redesign
- Clinical Trial Software Implementation
- Others
By Deployment Model
- On Premises
- Cloud
By Organization Size
- Small and Medium Sized
- Large Sized
By Application
- Recruitment Management
- Quality Assurance
- Site Management
- Financial Management
- Regulatory Compliance Management
- Data Management
- Patient Management
- Reporting and Analytics
- Safety and Adverse Event Management
- Others
By End User
- Biotechnology Companies
- Contract Research Organizations
- Healthcare Device Manufacturers
- Others
By Region
- North America (U.S., Canada, Mexico, Rest of North America)
- Europe (France, The UK, Spain, Germany, Italy, Nordic Countries (Denmark, Finland, Iceland, Sweden, Norway), Benelux Union (Belgium, The Netherlands, Luxembourg), Rest of Europe)
- Asia Pacific (China, Japan, India, New Zealand, Australia, South Korea, Southeast Asia (Indonesia, Thailand, Malaysia, Singapore, Rest of Southeast Asia), Rest of Asia Pacific)
- Middle East & Africa (Saudi Arabia, UAE, Egypt, Kuwait, South Africa, Rest of Middle East & Africa)
- Latin America (Brazil, Argentina, Rest of Latin America)
The Niche Research approach encompasses both primary and secondary research methods to provide comprehensive insights. While primary research is the cornerstone of our studies, we also incorporate secondary research sources such as company annual reports, premium industry databases, press releases, industry journals, and white papers.
Within our primary research, we actively engage with various industry stakeholders, conducting paid interviews and surveys. Our meticulous analysis extends to every market participant in major countries, allowing us to thoroughly examine their portfolios, calculate market shares, and segment revenues.
Our data collection primarily focuses on individual countries within our research scope, enabling us to estimate regional market sizes. Typically, we employ a bottom-up approach, meticulously tracking trends in different countries. We analyze growth drivers, constraints, technological innovations, and opportunities for each country, ultimately arriving at regional figures.Our process begins by examining the growth prospects of each country. Building upon these insights, we project growth and trends for the entire region. Finally, we utilize our proprietary model to refine estimations and forecasts.
Our data validation standards are integral to ensuring the reliability and accuracy of our research findings. Here’s a breakdown of our data validation processes and the stakeholders we engage with during our primary research:
- Supply Side Analysis: We initiate a supply side analysis by directly contacting market participants, through telephonic interviews and questionnaires containing both open-ended and close-ended questions. We gather information on their portfolios, segment revenues, developments, and growth strategies.
- Demand Side Analysis: To gain insights into adoption trends and consumer preferences, we reach out to target customers and users (non-vendors). This information forms a vital part of the qualitative analysis section of our reports, covering market dynamics, adoption trends, consumer behavior, spending patterns, and other related aspects.
- Consultant Insights: We tap into the expertise of our partner consultants from around the world to obtain their unique viewpoints and perspectives. Their insights contribute to a well-rounded understanding of the markets under investigation.
- In-House Validation: To ensure data accuracy and reliability, we conduct cross-validation of data points and information through our in-house team of consultants and utilize advanced data modeling tools for thorough verification.
The forecasts we provide are based on a comprehensive assessment of various factors, including:
- Market Trends and Past Performance (Last Five Years): We accurately analyze market trends and performance data from preceding five years to identify historical patterns and understand the market’s evolution.
- Historical Performance and Growth of Market Participants: We assess the historical performance and growth trajectories of key market participants. This analysis provides insights into the competitive landscape and individual company strategies.
- Market Determinants Impact Analysis (Next Eight Years): We conduct a rigorous analysis of the factors that are projected to influence the market over the next eight years. This includes assessing both internal and external determinants that can shape market dynamics.
- Drivers and Challenges for the Forecast Period:Identify the factors expected to drive market growth during the forecast period, as well as the challenges that the industry may face. This analysis aids in deriving an accurate growth rate projection.
- New Acquisitions, Collaborations, or Partnerships: We keep a close watch on any new acquisitions, collaborations, or partnerships within the industry. These developments can have a significant impact on market dynamics and competitiveness.
- Macro and Micro Factors Analysis:A thorough examination of both macro-level factors (e.g., economic trends, regulatory changes) and micro-level factors (e.g., technological advancements, consumer preferences) that may influence the market during the forecast period.
- End-User Sentiment Analysis: To understand the market from the end-user perspective, we conduct sentiment analysis. This involves assessing the sentiment, preferences, and feedback of the end-users, which can provide valuable insights into market trends.
- Perspective of Primary Participants: Insights gathered directly from primary research participants play a crucial role in shaping our forecasts. Their perspectives and experiences provide valuable qualitative data.
- Year-on-Year Growth Trend: We utilize a year-on-year growth trend based on historical market growth and expected future trends. This helps in formulating our growth projections, aligning them with the market’s historical performance.
Research process adopted by TNR involves multiple stages, including data collection, validation, quality checks, and presentation. It’s crucial that the data and information we provide add value to your existing market understanding and expertise. We have also established partnerships with business consulting, research, and survey organizations across regions and globally to collaborate on regional analysis and data validation, ensuring the highest level of accuracy and reliability in our reports.









