Global CRO for Biotech Market By Service Type, By Therapeutic Area, By End User, By Region & Segmental Insights Trends and Forecast, 2024 – 2034
- Industry: Healthcare
- Report ID: TNR-110-1127
- Number of Pages: 420
- Table/Charts : Yes
- June, 2024
- Base Year : 2024
- No. of Companies : 10+
- No. of Countries : 29
- Views : 10308
- Covid Impact Covered: Yes
- War Impact Covered: Yes
- Formats : PDF, Excel, PPT
The CRO for biotech market is experiencing robust growth, driven by several key trends and growth drivers. Increasing R&D expenditures, rising complexity in clinical trials, and the push for personalized medicine are major catalysts. Biotech firms are outsourcing to CROs to leverage their specialized expertise, reduce costs, and accelerate time-to-market. Additionally, advancements in technology, such as AI and big data analytics, are enhancing trial efficiency and data accuracy. The globalization of clinical trials is another significant trend, offering access to diverse patient populations. Regulatory pressures and the need for stringent compliance also drive biotech companies to partner with CROs, ensuring adherence to international standards and facilitating smoother market entry.
In terms of revenue, the global CRO for biotech market was worth US$ 87.65 Bn in 2023 and is anticipated to witness a CAGR of 13.5% during 2024 – 2034.

Trends in the Global CRO for Biotech Market
- Increasing Adoption of AI and Big Data Analytics- These technologies are revolutionizing how clinical trials are designed and conducted. AI enables predictive modeling, identifying potential trial outcomes and optimizing patient recruitment. Big data analytics, on the other hand, allows for the handling and analysis of vast amounts of data, providing deeper insights into patient responses and drug efficacy. This technological integration enhances trial efficiency, reduces costs, and accelerates time-to-market for new therapies. Moreover, the ability to analyze real-world evidence and patient data ensures more personalized and precise treatment options, aligning with the growing emphasis on personalized medicine in the biotech industry.
- Globalization of Clinical Trials- CROs are increasingly conducting trials across multiple countries to access diverse patient populations and meet regulatory requirements in different regions. This approach not only broadens the demographic reach of clinical studies but also accelerates patient recruitment, especially for rare diseases. Global trials help in understanding regional variations in drug efficacy and safety, which is crucial for market approval. Additionally, conducting trials in various regions can potentially lower costs and streamline the approval process in international markets. This trend is driven by the need for comprehensive data to support regulatory submissions worldwide, making the role of CROs vital in navigating the complex global regulatory landscape.
Global CRO for Biotech Market Revenue & Forecast, (US$ Million), 2016 – 2034
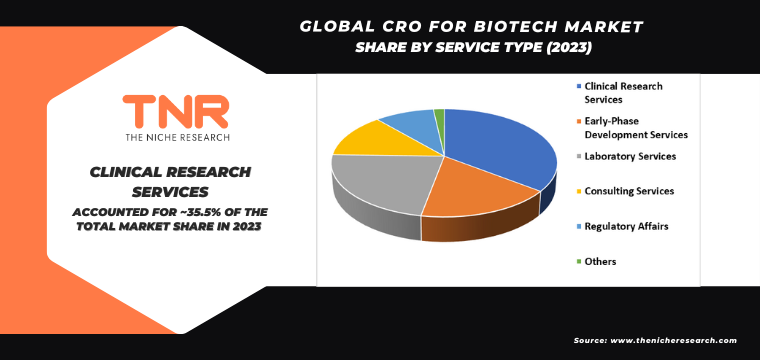
Clinical research services by service type dominated the global CRO for biotech market during the forecast period. This dominance is attributed to the increasing complexity of clinical trials and the need for specialized expertise in managing them. Clinical research services encompass a wide range of activities, including trial design, patient recruitment, data management, and regulatory compliance. Outsourcing these services to CROs allows biotech companies to leverage advanced technologies, streamline operations, and focus on core research activities. Additionally, the globalization of clinical trials necessitates robust clinical research services to ensure regulatory adherence and efficient trial execution across diverse geographic regions.
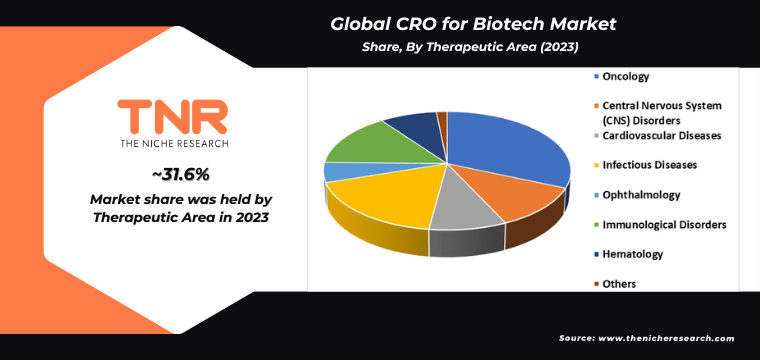
Infectious diseases segment by therapeutic area has gained significant popularity in recent years and is anticipated to grow the fastest over the forecast timeline. This surge is driven by the urgent need for new treatments and vaccines, highlighted by global health crises such as the COVID-19 pandemic. Increased funding and attention from both public and private sectors are fueling research and development in this area. Advances in biotechnology, such as mRNA technology, are accelerating the discovery and development of innovative therapies. Additionally, the rise in antimicrobial resistance and emerging infectious threats further underscores the importance of this rapidly growing segment within the CRO for biotech market.
In 2023, pharmaceutical and biopharmaceutical companies held the largest revenue share in the global market, accounting for 43.3%. This significant share is attributed to the growing trend of outsourcing clinical research to specialized CROs. By partnering with CROs, these companies can access advanced technologies, streamline clinical trials, and reduce operational costs. The increasing complexity of drug development, coupled with stringent regulatory requirements, further drives the reliance on CROs. Additionally, the push for innovative therapies and personalized medicine has led to more extensive and diverse clinical studies, solidifying the crucial role of CROs in the pharmaceutical and biopharmaceutical sectors.

By region, Asia Pacific is expected to emerge as the fastest growing region in the CRO for biotech market in 2023. This rapid growth is fueled by several factors, including cost-effective clinical trial operations, a large and diverse patient pool, and increasing investments in healthcare infrastructure. Countries like China and India are becoming key hubs for clinical research due to favorable regulatory environments and government support. Additionally, the region’s growing biotech industry and advancements in medical technology are attracting global biotech companies to outsource their clinical research activities to Asia Pacific CROs, is further driving market expansion in this region.

Competitive Landscape
The competitive landscape of the CRO market for biotech is characterized by the presence of several key players striving for market share through mergers, acquisitions, and strategic partnerships. Leading firms like Charles River Laboratories, LabCorp, and Parexel dominate due to their extensive service portfolios and global reach. Innovations in clinical trial technology and expanding service offerings are critical for maintaining competitive advantage and meeting the evolving needs of biotech companies.
Some of the players operating in the CRO for biotech market are
- Avance Clinical
- Bioagile
- Charles River Laboratories
- Firma Clinical Research
- Frontage Labs
- Geneticist In.
- Laboratory Corporation of America
- Novotech
- Parexel International
- SanaClis s.r.o.
- Vial
- WuXi AppTec
- Other Industry Participants
Global CRO for Biotech Market Scope
| Report Specifications | Details |
| Market Revenue in 2023 | US$ 87.56 Bn |
| Market Size Forecast by 2034 | US$ 352.96 Bn |
| Growth Rate (CAGR) | 13.5% |
| Historic Data | 2016 – 2022 |
| Base Year for Estimation | 2023 |
| Forecast Period | 2024 – 2034 |
| Report Inclusions | Market Size & Estimates, Market Dynamics, Competitive Scenario, Trends, Growth Factors, Market Determinants, Key Investment Segmentation, Product/Service/Solutions Benchmarking |
| Segments Covered | By Service Type, By Therapeutic Area, By End User, By Region |
| Regions Covered | North America, Europe, Asia Pacific, Middle East & Africa, Latin America |
| Countries Covered | U.S., Canada, Mexico, Rest of North America, France, The UK, Spain, Germany, Italy, Nordic Countries (Denmark, Finland, Iceland, Sweden, Norway), Benelux Union (Belgium, The Netherlands, Luxembourg), Rest of Europe, China, Japan, India, New Zealand, Australia, South Korea, Southeast Asia (Indonesia, Thailand, Malaysia, Singapore, Rest of Southeast Asia), Rest of Asia Pacific, Saudi Arabia, UAE, Egypt, Kuwait, South Africa, Rest of Middle East & Africa, Brazil, Argentina, Rest of Latin America |
| Key Players | Avance Clinical, Bioagile, Charles River Laboratories, Firma Clinical Research, Frontage Labs, Geneticist In., Laboratory Corporation of America, Novotech, Parexel International, SanaClis s.r.o., Vial, WuXi AppTec |
| Customization Scope | Customization allows for the inclusion/modification of content pertaining to geographical regions, countries, and specific market segments. |
| Pricing & Procurement Options | Explore purchase options tailored to your specific research requirements |
| Contact Details | Consult With Our Expert
Japan (Toll-Free): +81 663-386-8111 South Korea (Toll-Free): +82-808- 703-126 Saudi Arabia (Toll-Free): +966 800-850-1643 United Kingdom: +44 753-710-5080 United States: +1 302-232-5106 E-mail: askanexpert@thenicheresearch.com
|
Global CRO for Biotech Market
By Service Type
- Clinical Research Services
- Phase I
- Phase II
- Phase III
- Phase IV
- Early-Phase Development Services
- Drug Discovery
- Bioequivalence & Bioavailability
- Pharmacodynamics
- Pharmacokinetics
- Preclinical Development
- Laboratory Services
- Bioanalytical Services
- Analytical Testing
- PBMC Processing
- Biomarker Discovery
- Consulting Services
- Regulatory Consulting
- Clinical Development Consulting
- PMDA Consultation
- Medical Writing
- Quality Assurance Consulting
- Regulatory Affairs
- Others
By Therapeutic Area
- Oncology
- Breast Cancer
- Colorectal Cancer
- Lung Cancer
- Prostate Cancer
- Others
- Central Nervous System (CNS) Disorders
- Cardiovascular Diseases
- Infectious Diseases
- Ophthalmology
- Immunological Disorders
- Hematology
- Others
By End User
- Pharmaceutical and Biopharmaceutical Companies
- Academic and Research Institutes
- Others
By Region
- North America (U.S., Canada, Mexico, Rest of North America)
- Europe (France, The UK, Spain, Germany, Italy, Nordic Countries (Denmark, Finland, Iceland, Sweden, Norway), Benelux Union (Belgium, The Netherlands, Luxembourg), Rest of Europe)
- Asia Pacific (China, Japan, India, New Zealand, Australia, South Korea, Southeast Asia (Indonesia, Thailand, Malaysia, Singapore, Rest of Southeast Asia), Rest of Asia Pacific)
- Middle East & Africa (Saudi Arabia, UAE, Egypt, Kuwait, South Africa, Rest of Middle East & Africa)
- Latin America (Brazil, Argentina, Rest of Latin America)
Report Layout:
Table of Contents
Note: This ToC is tentative and can be changed according to the research study conducted during the course of report completion.
**Exclusive for Multi-User and Enterprise User.
Global CRO for Biotech Market
By Service Type
- Clinical Research Services
- Phase I
- Phase II
- Phase III
- Phase IV
- Early-Phase Development Services
- Drug Discovery
- Bioequivalence & Bioavailability
- Pharmacodynamics
- Pharmacokinetics
- Preclinical Development
- Laboratory Services
- Bioanalytical Services
- Analytical Testing
- PBMC Processing
- Biomarker Discovery
- Consulting Services
- Regulatory Consulting
- Clinical Development Consulting
- PMDA Consultation
- Medical Writing
- Quality Assurance Consulting
- Regulatory Affairs
- Others
By Therapeutic Area
- Oncology
- Breast Cancer
- Colorectal Cancer
- Lung Cancer
- Prostate Cancer
- Others
- Central Nervous System (CNS) Disorders
- Cardiovascular Diseases
- Infectious Diseases
- Ophthalmology
- Immunological Disorders
- Hematology
- Others
By End User
- Pharmaceutical and Biopharmaceutical Companies
- Academic and Research Institutes
- Others
By Region
- North America (U.S., Canada, Mexico, Rest of North America)
- Europe (France, The UK, Spain, Germany, Italy, Nordic Countries (Denmark, Finland, Iceland, Sweden, Norway), Benelux Union (Belgium, The Netherlands, Luxembourg), Rest of Europe)
- Asia Pacific (China, Japan, India, New Zealand, Australia, South Korea, Southeast Asia (Indonesia, Thailand, Malaysia, Singapore, Rest of Southeast Asia), Rest of Asia Pacific)
- Middle East & Africa (Saudi Arabia, UAE, Egypt, Kuwait, South Africa, Rest of Middle East & Africa)
- Latin America (Brazil, Argentina, Rest of Latin America)
The Niche Research approach encompasses both primary and secondary research methods to provide comprehensive insights. While primary research is the cornerstone of our studies, we also incorporate secondary research sources such as company annual reports, premium industry databases, press releases, industry journals, and white papers.
Within our primary research, we actively engage with various industry stakeholders, conducting paid interviews and surveys. Our meticulous analysis extends to every market participant in major countries, allowing us to thoroughly examine their portfolios, calculate market shares, and segment revenues.
Our data collection primarily focuses on individual countries within our research scope, enabling us to estimate regional market sizes. Typically, we employ a bottom-up approach, meticulously tracking trends in different countries. We analyze growth drivers, constraints, technological innovations, and opportunities for each country, ultimately arriving at regional figures.Our process begins by examining the growth prospects of each country. Building upon these insights, we project growth and trends for the entire region. Finally, we utilize our proprietary model to refine estimations and forecasts.
Our data validation standards are integral to ensuring the reliability and accuracy of our research findings. Here’s a breakdown of our data validation processes and the stakeholders we engage with during our primary research:
- Supply Side Analysis: We initiate a supply side analysis by directly contacting market participants, through telephonic interviews and questionnaires containing both open-ended and close-ended questions. We gather information on their portfolios, segment revenues, developments, and growth strategies.
- Demand Side Analysis: To gain insights into adoption trends and consumer preferences, we reach out to target customers and users (non-vendors). This information forms a vital part of the qualitative analysis section of our reports, covering market dynamics, adoption trends, consumer behavior, spending patterns, and other related aspects.
- Consultant Insights: We tap into the expertise of our partner consultants from around the world to obtain their unique viewpoints and perspectives. Their insights contribute to a well-rounded understanding of the markets under investigation.
- In-House Validation: To ensure data accuracy and reliability, we conduct cross-validation of data points and information through our in-house team of consultants and utilize advanced data modeling tools for thorough verification.
The forecasts we provide are based on a comprehensive assessment of various factors, including:
- Market Trends and Past Performance (Last Five Years): We accurately analyze market trends and performance data from preceding five years to identify historical patterns and understand the market’s evolution.
- Historical Performance and Growth of Market Participants: We assess the historical performance and growth trajectories of key market participants. This analysis provides insights into the competitive landscape and individual company strategies.
- Market Determinants Impact Analysis (Next Eight Years): We conduct a rigorous analysis of the factors that are projected to influence the market over the next eight years. This includes assessing both internal and external determinants that can shape market dynamics.
- Drivers and Challenges for the Forecast Period:Identify the factors expected to drive market growth during the forecast period, as well as the challenges that the industry may face. This analysis aids in deriving an accurate growth rate projection.
- New Acquisitions, Collaborations, or Partnerships: We keep a close watch on any new acquisitions, collaborations, or partnerships within the industry. These developments can have a significant impact on market dynamics and competitiveness.
- Macro and Micro Factors Analysis:A thorough examination of both macro-level factors (e.g., economic trends, regulatory changes) and micro-level factors (e.g., technological advancements, consumer preferences) that may influence the market during the forecast period.
- End-User Sentiment Analysis: To understand the market from the end-user perspective, we conduct sentiment analysis. This involves assessing the sentiment, preferences, and feedback of the end-users, which can provide valuable insights into market trends.
- Perspective of Primary Participants: Insights gathered directly from primary research participants play a crucial role in shaping our forecasts. Their perspectives and experiences provide valuable qualitative data.
- Year-on-Year Growth Trend: We utilize a year-on-year growth trend based on historical market growth and expected future trends. This helps in formulating our growth projections, aligning them with the market’s historical performance.
Research process adopted by TNR involves multiple stages, including data collection, validation, quality checks, and presentation. It’s crucial that the data and information we provide add value to your existing market understanding and expertise. We have also established partnerships with business consulting, research, and survey organizations across regions and globally to collaborate on regional analysis and data validation, ensuring the highest level of accuracy and reliability in our reports.









